A Test of Cure Using a New Molecular Diagnostic Approach for Bacterial Vagino- sis in Infected Women Treated with Dequalinium Chloride Vaginal Tablets
Received Date: May 23, 2020 Accepted Date: June 17, 2020 Published Date: June 24, 2020
doi: 10.17303/jwhg.2020.7.205
Citation: Per-Göran Larsson (2020) A Test of Cure Using a New Molecular Diagnostic Approach for Bacterial Vaginosis in Infected Women Treated with Dequalinium Chloride Vaginal Tablets. J Womens Health Gyn 7: 1-12.
Abstract
Background: Consensus has not yet been reached on the optimal method for diagnosing bacterial vaginosis. Different molecular diagnostic methods for the diagnosis of bacterial vaginosis have been introduced as alternatives to traditional clinical diagnostic methods. We have previously tested our molecular test for the diagnosis of BV with an excellent agreement with the modified Hay/Ison criteria, with a kappa coefficient value of 0.87 (0.76–0.99). None of the molecular diagnostic methods have previously been utilized as a test of cure.
Material and Methods: Fifty women diagnosed with bacterial vaginosis and treated with dequalinium chloride vaginal tablets were re-examined one, two, three, and 12 months after their primary treatment. On all occasions, the bacterial vaginosis status of the women was examined using modified Hay/Ison criteria with concurrent molecular diagnostic testing using polymerase chain reaction analysis for the amplification of specific DNA targets for Gardnerella vaginalis, Atophobiumvaginae, Leptotrichiaspp., Megasphaeraspp., Mobiluncusspp., BVAB2, and Lactobacillus spp.
Results: The bacterial vaginosis cure rate after treatment with dequalinium chloride vaginal tablets was only 37% one month following treatment; after 12 months, only 13% of the patients remained cured. However, consecutive treatment with clindamycin resulted in a cure rate higher than expected, suggesting that the combination of the two different treatments may have a synergistic effect. The molecular diagnostic test had a kappa index of 0.86 relative to microscopy using modified Hay/Ison criteria for the clinical diagnosis of bacterial vaginosis.
Conclusions: This is the first study to utilize a molecular diagnostic test as a test of cure for bacterial vaginosis. The Nugent score is a reliable method for diagnosing bacterial vaginosis, but similar to traditional clinical diagnostic methods, it is not equally reliable when utilized as a test of cure. This study demonstrated that molecular diagnostic testing can be used as a test of cure and this is the first molecular test that can do that. Treatment with dequalinium chloride vaginal tablets for 6 days had a cure rate lower than expected; only 37% of patients were considered cured one month after treatment.
Trial registration: Clinical trial registration 14 August 2019 # NCT04047482, retrospectively registered
Keywords: Bacterial vaginosis; vaginitis; treatment; polymerase chain reaction; molecular diagnostic techniques.
Key Message: The molecular test can be used both for the primary diagnosis of bacterial vaginosis and also as a test of cure. This is very important as many patients with bacterial vaginosis often experience a relapse.
Abbreviations: BV: Bacterial Vaginosis; MDA: Medical Products Agency
Background
Bacterial vaginosis (BV) is a common cause of vaginal inflammation and discomfort in women of reproductive age. Although no single etiological factor has been identified, molecular studies have shown a change in the vaginal microbiota is associated with BV infection, [1-4] where the normally lactobacilli-dominant vaginal flora is overtaken by anaerobic bacteria such as Gardnerella vaginalis, Atopobiumvaginae, Mobiluncus spp. and Bacteroides spp.
BV is commonly clinically diagnosed with Amsel's scriteria [5] or by microscopy using either Nugent’s criteria [6] or the Hay/Ison criteria [7,8]. These clinical diagnostic methods are all based on subjective assessment and microscopy evaluation, which require highly skilled personnel. There is thus a growing need to validate easier and more objective methods than those currently used. Different molecular diagnostic methods for the diagnosis of BV have been introduced [9-12] Recently. Schwebke, et al. [13] published a molecular diagnostic method using the BD MAX™ Vaginal Panel from Becton, Dickinson, and Company, which showed promising results with a more reliable diagnosis than traditional clinical diagnostic methods, such as Amsel’s criteria. However, whether molecular diagnostic methods are suitable for use as a “test of cure” has not yet been investigated. Nugent's criteria for the diagnosis of BV is a good diagnostic method, but it has not been equally reliable when used as a test of cure [14].
Numerous studies investigating the treatment of BV have been published [15], but there is still no consensus on how treatments should be evaluated. First, the time point for when a test of cure is performed varies widely between studies, ranging from the day after the end of treatment up to 3 weeks after the completion of treatment, or after the following menstruation [16].
The gold standard for the diagnosis of BV is the Amsel criteria [5]. According to Amsel’s criteria, to confirm a diagnosis of BV, three out of four clinical criteria should be fulfilled. These criteria include typical homogeneous vaginal discharge, an elevated vaginal pH >4.5, a positive potassium hydroxide test (whiff test), and the presence of clue cells. These criteria can be considered subjective, and their interpretation can differ among clinicians. Across different studies, there has also been a difference in the cutoff value used for clue cells. Eschenbach, et al. [17] concluded that most BV-infected patients possess more than 20% clue cells; therefore, this cutoff value has been used in several treatment studies to determine a BV diagnosis. Therefore, to fulfill the diagnosis of BV, the patient must have more than 20% clue cells. As a consequence, women with fewer than 20% clue cells could be considered cured of their BV infection, which is not consistent with the original works by both Amsel and Gardner, [18] where the term “clue cells” was originally introduced.
The most commonly accepted laboratory diagnostic test for BV considers Nugent’s criteria [6]. A swab is obtained from the lateral vaginal wall and is rolled onto a glass slide. The smears are subsequently Gram stained. The number of Lactobacillus morphotypes is calculated; whereby the presence of more than 30 lactobacilli per vision field gives a score of 0, 5–30 lactobacilli gives a score of 1, 1–4 lactobacilli gives a score of 2, an average score of fewer than 1 lactobacilli gives a score of 3, and no lactobacilli observed per visual field gives a score of 4. Gardnerella-like bacteria are scored in a similar manner but in the reverse order. In addition, the presence of curved gram-variable rods (Mobiluncus spp. morphotypes) will result in the addition of 1–2 points to the score depending on the number observed. A total score of 0–3 is considered normal, a score of 4–6 is considered intermediate, and a score of 7–10 is considered a conclusive diagnosis of BV
Nugent’s method has some disadvantages. First, the smears are scored by quantification of the different vaginal morphotypes, which requires an experienced laboratory technician as well as considerable time and skill [19]. Second, quantification of the morphotypes also depends on the visual field of the microscope, which may differ by more than 300% between different microscopes, [14] particularly in modern laboratory microscopes. According to Nugent’s criteria, an average of 30 Gardnerella morphotypes results in a score of 4. The use of a different microscope with an area that is 300% larger should require three times more bacteria i.e., a total of 90 Gardnerella morphotypes to achieve the same score. This has been recognized among pathologists assessing the histological grades of breast cancer, the mitotic count's per microscopic field is calculated. The mitotic activity is assessed in a minimum of 10 fields. Up to nine mitoses, per10 fields give a score of 1, 10–19 mitoses give a score of 2, and more than 20 mitoses a score of 3, based on a microscope with a field area of 0.274 mm2. This scoring system can then easily be adapted to other microscopes with different field areas using a graph that has been constructed for this purpose and which compensates for differences in microscopic area [20].
A simpler method was described by Hay, et al. [7,8] in which the vaginal flora is divided into the following categories: normal (many lactobacilli morphotypes—few Gardnerella morphotypes), intermediate BV (equal numbers of lactobacilli and Gardnerella morphotypes), and BV (few lactobacilli and many Gardnerella morphotypes).
The Hay/Ison criteria were originally developed using Gram-stained smears by oil immersion at a magnification of 1000x; however, because this criterion assesses the type of flora and does not indicate the number of individual bacteria, as in Nugent’s criteria, it is possible to use these criteria with nonstained smears at relatively low magnification. A modification of the Hay/Ison criteria has recently been validated [21]. According to the modified Hay/Ison criteria, vaginal samples are first airdried. This allows the samples to be transported and saved for later investigation without affecting the sample’s integrity. Upon arrival to the laboratory, a drop of saline is added to each sample for rehydration, and a coverslip is placed over the sample in preparation for analysis by microscopy.
Dequalinium chloride vaginal tablets have recently been registered in the Nordic countries after approval from the Swedish MDA. Dequalinium chloride is not an antibiotic but an antiseptic and has been used for many years, especially in Eastern Europe. However, one English-language publication from a study in Germany claimed that treatment with dequalinium chloride vaginal tablets was as effective as treatment with clindamycin vaginal cream [22].
The primary goal of this study was to investigate the cure rate after treatment with dequalinium chloride vaginal tablets in an open-label study with consecutive patients. A secondary goal was to investigate whether a molecular diagnostic test for BV may be a reliable method for utilization as a test of cure capable of also identifying infection relapses.
Methods
Women were recruited from local maternity and youth clinics in Skaraborg county, Sweden. Eligibility for inclusion in the study was based on the following criteria: women who were 18 years and older, who experienced regular menstruation, who used contraception, who were not planning to become pregnant, who tested negative for Chlamydia trachomatis and Neisseria gonorrhea, using standard PCR method with Aptima Combo 2 CT/, Hologic inc and who took no other antibiotic treatment during the last month before or during the commencement of the study’s treatment.
Clinical diagnostic test for BV
A vaginal sample was collected by a midwife from women presenting with symptoms of a malodorous vaginal discharge. Each sample was collected from the lateral fornix and transferred onto a microscope slide, air-dried, and sent to Skaraborgs Hospital’s Gynaecology Department in Skövde, Sweden. All vaginal samples were examined using a phase-contrast microscope at a magnification of 400x according to the modified Hay/Ison criteria for the diagnosis of BV [21]. The vaginal flora was divided into the following three categories depending on the relative number of Lactobacillus morphotypes compared to Gardnerella morphotypes: normal (grade 1), intermediate (grade 2), and BV (grade 3). At least four fields were evaluated in all the vaginal samples.
Treatment
Women clinically diagnosed with BV were offered treatment with dequalinium chloride vaginal tablets (Fluomizin® or in Nordic countries Donaxyl®). Each participant was asked to collect a vaginal sample by themselves using FLOQSwabs™ (Copan, Brescia, Italy) after their next menstruation following treatment. The swab was rolled on a microscope slide that was subsequently allowed to air-dry. The swab and the microscope slide were sent to Skaraborgs Hospital’s Gynaecology Department in Skövde.
During the follow-up period, all the women were contacted by the research nurse, and if the women were clinically determined to be cured according to the results of the microscope i.e, evaluation using the modified Hay/Ison criteria grad 1 and 2, the patient was asked to deliver a new self-collected vaginal sample after their next menstruation had stopped. If BV persisted, and the patient did not have a regular partner the patient was offered treatment with 2% clindamycin vaginal cream administered daily for seven days, and further follow-up with a new vaginal sample after the subsequent menstruation was conducted. If the patient had a regular sexual partner, both she and her partner were prescribed treatment with 300 mg clindamycin administered orally twice daily for seven days. New vaginal samples were requested three, four, six, and 12 months after the primary treatment. Because not all patients send in all samples the results are given for two, three, and 12 months. All women who had to send in samples had been treated either with dequalinium chloride vaginal tablets once or with clindamycin. The investigation of the microscopy was blinded to the previous findings.
Molecular diagnostic test for BV
The vaginal samples were sent to Dynamic Code’s laboratory in Linköping, Sweden, for the molecular diagnosis of BV within one week of the sample's arrival to the Gynaecology Department at Skaraborgs Hospital, Skövde. The samples were analyzed upon arrival at the laboratory, and the results of the molecular diagnostic test were not disclosed until all patients had each delivered their 12-month sample. The molecular diagnostic test involved real-time polymerase chain reaction analysis with the amplification of specific DNA targets for Gardnerella vaginalis, Atophobiumvaginae, Leptotrichiaspp., Megasphaeraspp., Mobiluncusspp., BVAB2, lactobacilli and Candida spp. Dynamic Code’s proprietary algorithm produced either a positive or a negative BV result depending on the presence and concentration of each of the aforementioned bacteria relative to lactobacilli. The laboratory is accredited according to ISO/IEC 17025. The molecular test has been described more in detail in an earlier article [23]. The article showed that there is an excellent agreement between the molecular test and wet mount microscopy of vaginal swab samples using modified Hay/Ison criteria for 288 women seeking therapeutic abortion with a kappa coefficient value of 0.87 (0.76–0.99). This gives a sensitivity of 0.91 (0.82-0.95), a specificity of 0.97(0.93-0.98). Before this test can be recommended it has to be investigated that it can also be used as a test of cure [23].
Molecular diagnostic tests were performed on patient samples from patients who had been treated either solely with dequalinium chloride vaginal tablets or in combination with clindamycin vaginal cream. The subsequent tests performed one month, two months, three months, and 12 months following the patient's primary treatment could thus be used as comparisons with the clinical diagnostic test to determine the reliability of the method as a test of cure. For patients who were regarded as cured based on the results of their clinical diagnostic tests, new tests were performed to observe whether the negative result persisted after another month.
Statistical analyses
The cure rate, mean, sensitivity, specificity, positive predictive value, and negative predictive value were calculated according to standard equations. The degree of agreement between the two methods was estimated by Cohen’s kappa coefficient and the confidence intervals were calculated [24]. Not all patients collected a vaginal fluid sample every month, but all who sampled at least once were included in the analysis. In case of missing data during the follow-up, the procedure "last observation carried forward" (LOCF) was used.
Ethical Approval
The study was approved by the Regional Ethical Review Board (EPN) in Gothenburg (DNR 160-15) 15 April 2015. Informed consent was obtained from all the participants before participation.
Clinical trial registration was done on 08/14/2019 # NCT04047482, retrospectively registered.
Results
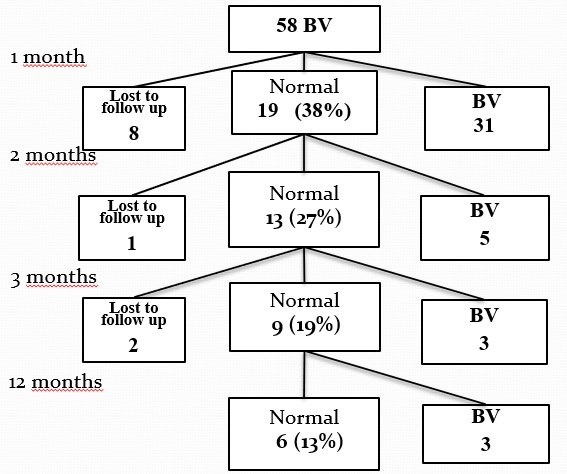
A total of 58 women were recruited for the study, with 50 women completing the study. The women were offered treatment with dequalinium chloride vaginal tablets for seven days. Eight women exclude from the study during the first month of follow-up, mainly due to chlamydia infection. The mean age of the women included in the study was 25.8 years. The cure rate after one month was only 37% (Figure 1). During the second month, another participant dropped out, and five women had a relapse of BV. After 12 months, only six of the 47 women examined were still cured of BV, resulting in a cure rate of 12.8%. The mean age of the six cured patients was 32.6 years. Of the cured patients, one had a regular sexual partner, two had no regular sexual partner, and three did not answer the question. The 32 women with BV after the first menstruation were offered treatment with either oral clindamycin 300 mg BID for seven days (n=19) or vaginal clindamycin 2% for seven (n=12) days depending on whether the patient had a regular sexual partner. During follow-up, three women, one with a regular partner and two without, dropped out. In the regular partner group, 16 out of 18 women (89%) were cured of BV one month after treatment. Similarly, in the group without regular sexual partners, eight out of 10 women (80%) were cured of BV
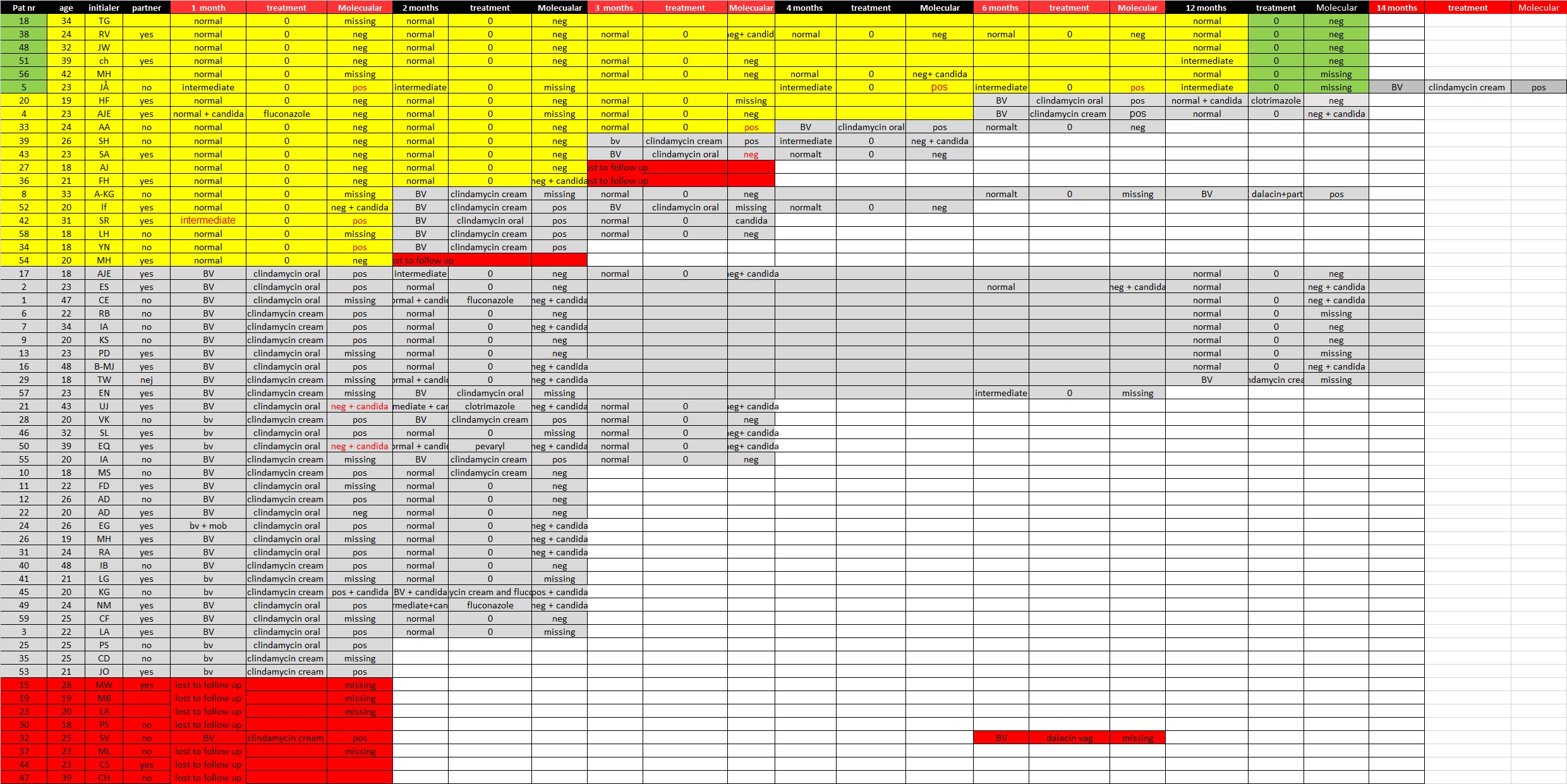
Of the 150 samples submitted, 116 were also analyzed for the molecular diagnosis of BV (Table 1). The modified Hay/ Ison classification classifies the samples as normal, intermediate, or BV positive, whereas the molecular diagnostic test gives a positive or a negative result for BV. For comparison, the normal and intermediate microscopic samples were merged into a “nonBV” group. Ninety-six samples showed concordant results, and 10 samples showed discordant results, resulting in a kappa index of 0.80. All samples with discordant results were re-evaluated.
There were six samples that were regarded as BV positive using the molecular diagnostic test and as normal or intermediate with microscopy. Three of the tests that were classified as intermediate according to the microscopy analysis of rehydrated wet smears were all from the same patient collected at one, three, and six months. At 12 months she was still intermediate but that time we did not have any molecular test. She came with a clinical relapse at 14 months, However, the microscopy test result agreed with the molecular diagnostic test result and classified the sample as positive for BV. The remaining three samples that were positive for BV by the molecular diagnostic test came from women who presented BV-positive samples by microscopy a month later. After re-evaluation, two samples that had short lactobacilli and the samples were very thin samples containing some interfering blood. Thin samples with few epithelial cells are difficult to classify and they were classified as BV positive.
Four samples were classified as negative for BV by the molecular diagnostic test and positive for BV by microscopy. After re-evaluation, one sample was reclassified as normal. Two of these discordant results were associated with clinical Candida infections that were treated. Both of these women also had positive results for Candida with the molecular diagnostic test. The kappa value was recalculated as 0.86 (Table 2). See also the cart from all patients (Figure 2).
Discussion
The cure rate with dequalinium chloride in the present study was much lower than that reported in the study by Weisenbacher, et al. [22], which concluded that the dequalinium chloride treatment cure rate was equivalent to that of vaginal clindamycin treatment. One major difference between these studies was the criteria for a cure. Weisenbacher, et al. [22] used the criteria in which the presence of less than 20% clue cells is consistent with the cure, which was introduced by Eschenbach, et al. [17] but has never been validated in comparison to Amsel’s criteria. [5].
Dequalinium chloride is not an antibiotic and will therefore not contribute to increasing antibiotic resistance, which is why the treatment is very popular in Scandinavian countries. However, in our study, it seemed that the cure rate was relatively low. Interestingly, the cure rate after treatment with clindamycin was higher than expected, with more than 80%. In an earlier study that used two out of three of Amsel's criteria (elevated pH, clue cells, and potassium hydroxide test) as a test of cure, the cure rate was 70% [15].In studies in which all three criteria had to be met for the patient to be considered cured, the cure rate fell below 50% [25, 26].A possible explanation for the relatively high cure rate observed in the present study is that the dequalinium chloride given before treatment with clindamycin affects the bio film in the vagina, [27] making the bacteria more susceptible to antibiotic treatment [28, 29]. If this proves to be true, a possible treatment regime could be dequalinium chloride administered daily for six days directly followed by a seven-day course of vaginal clindamycin.
The current study also shows that the diagnosis of BV using Dynamic Code’s algorithm works exceedingly well for both the primary diagnosis and as a test of cure. Of the discordant samples, a positive molecular diagnostic test result was always followed by a positive modified Hay/Ison test result for BV in a later follow-up, indicating that the molecular diagnostic test is able to detect a relapse of BV at an earlier stage than traditional clinical diagnostic methods.
It is also difficult to compare a test with three alternative test results with a test that has only two results. The intermediate samples are in a true intermediate (transitional) state between BV and normal, meaning that either the women are developing BV or are being cured of BV. We included the intermediate results in the normal or lactobacilli classification (non-BV group). Perhaps this was not accurate, as the six patients for which the molecular diagnostic test showed BV and the modified Hay/Ison test showed an intermediate result all were positive for BV on the following test. Therefore, one can speculate that those with intermediate results in our study were women who were developing BV.
The limitations of the study could be that modified Hay/ Ison criteria is not the gold standard for BV diagnosis. However, it has been validated to the standard Hay/Ison using Gramstained smears and the standard Hay/Ison method has been validated to Amsel's method. Amsel's method has a lot of subjectivity and is not easy to replicate. We, therefore, conclude that the diagnosis of BV should be correct. Other limitations it that, administration of the prescribed medication by the patient could not be controlled that she took all prescribed days of treatment.
Conclusion
This is the first study that has utilized a molecular diagnostic test as a test of cure for BV. Nugent’s scoring criteria is a very good clinical diagnostic method for diagnosing BV, but it has not been shown to be equally reliable when utilized as a test of cure. This study demonstrated that the molecular diagnostic test was not only a reliable method for BV diagnosis but could also be used as a test of cure. To our knowledge, this molecular test is the first test that can be used as a test of cure. Furthermore, the molecular diagnostic test could, with a high probability, detect a relapse at an earlier stage than traditional microscopy using the modified Hay/Ison score. The results from the current study support the potential utility of the molecular diagnostic test in the diagnosis of BV.
The efficacy of treatment with dequalinium chloride vaginal tablets was lower than expected, [22] with only 37% of patients considered to be cured one month after treatment. However, we have tried to treat patients with first dequalinium chloride vaginal tablets and then followed by vaginal clindamycin cream treatment for 7 days. With this regimen, we have now treated 78 new patients with an 89% cure rate after 2 months.
Declarations
Ethics approval and consent to participate
The study was approved by the Regional Ethical Review Board (EPN) in Gothenburg Box 401 405 30 Göteborg Sweden, University of Gothenburg, Box 100, SE-405 30 Gothenburg, SWEDEN (dnr 160-15) 15 April 2015. Verbal and signed informed consent were obtained from all the participants before participation.
Clinical trial registration 14 August 2019 # NCT04047482, retrospectively registered.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to individual privacy could be compromised but are available from the corresponding author on reasonable request.
Competing interests
P.G.L. has received speaking fees from Campus Pharma AB. This study was conducted in cooperation with Dynamic Code AB, a company with a commercial interest in marketing a reliable molecular diagnostic test for bacterial vaginosis. They provided all the materials needed for collecting vaginal smears and molecular diagnostic testing. M.F. is employed as a Senior Scientist and A.H. is employed as a Product Developer at Dynamic Code AB. K.B, I.V and J.L declare that they have no conflicts of interest.
Funding
This study was partly funded by the Skaraborgs Hospital FoU fund. This funding is for employees in the hospital to make resurge possible even in non-university hospitals.
Authors' contributions
All authors have been involved in the writing of the article. PGL is the main author, KB and JL have been designing the study and IV have administered the study, and AH and MF have also done the molecular analysis. All authors have read and approved the revised manuscript.
- Srinivasan S, Fredricks DN (2008) The human vaginal bacterial biota and bacterial vaginosis. Interdiscip Perspect Infect Dis 2008: 750479.
- Ferris MJ, Masztal A, Aldridge KE, Fortenberry JD, et al. (2004) Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis 4: 5.
- Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, et al. (2002) Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 185: 1307- 1313.
- Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM (2007) Targeted PCR for the detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol 45: 3270-3276.
- Amsel R, Totten PA, Spiegel CA, Chen K, Eschenbach DA, et al. (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 74: 14-22.
- Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 29: 297-301.
- Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J (1994) Abnormal bacterial colonization of the genital tract and subsequent preterm delivery and late miscarriage. BMJ. 308: 295-8.
- Ison CA, Hay PE (2002) Validation of a simplified grading of Gram-stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect 78: 413-415.
- Menard JP, Fenollar F, Henry M, Bretelle F, Raoult D (2008) Molecular quantification of Gardnerella vaginalis and Atopobiumvaginae loads to predict bacterial vaginosis. Clin Infect Dis. 47: 33-43.
- Cartwright CP, Lembke BD, Ramachandran K, Body BA, et al. (2012) Development and validation of a semiquantitative, multitarget PCR assay for diagnosis of bacterial vaginosis. J Clin Microbiol 50: 2321-2329.
- Shipitsyna E, Guschin A, Maximova A, Tseslyuk M, et al. (2008) Comparison of microscopy, culture and in-house PCR and NASBA assays for diagnosis of Neisseria gonorrhoeae in Russia. APMIS116: 133-138.
- Gaydos CA, Beqaj S, Schwebke JR, Lebed J, et al. (2017) Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstet Gynecol. 130: 181-9.
- Schwebke JR, Gaydos CA, Nyirjesy P, Paradis S, et al. (2018) Diagnostic Performance of a Molecular Test versus Clinician Assessment of Vaginitis. J Clin Microbiol. 56.
- Larsson PG, Carlsson B, Fahraeus L, Jakobsson T, Forsum U (2004) Diagnosis of bacterial vaginosis: the need for validation of microscopic image area used for scoring bacterial morphotypes. Sex Transm Infect. 80:63-67.
- Larsson PG, Forsum U (2005) Bacterial vaginosis--a disturbed bacterial flora and treatment enigma. APMIS. 113: 305- 316.
- Larsson PG (1995) Bacterial vaginosis treatment evaluation before one month is not accurate. Am J Obstet Gynecol. 172: 1638-1639.
- Eschenbach DA, Hillier S, Critchlow C, Stevens C, et al. (1988) Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 158: 819-828.
- Gardner HL, Dukes CD (1995) Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified as non-specific vaginitis. Am J Obstet Gynecol. 69: 962-976.
- Forsum U, Larsson PG, Spiegel C (2008) Scoring vaginal fluid smears for diagnosis of bacterial vaginosis: the need for quality specifications. APMIS. 116: 156-159.
- National Coordinating Group For Breast Screening Pathology (1995) Pathology reporting in breast cancer screening. (2nd edn). NHSBSP publications no 3 April.
- Larsson P-G, Breding K, Vikström I, Larsson J (2018) Validation of Hay/Ison Criteria for A Diagnosis of Bacterial Vaginosis Using an Air-Dried Wet Smear. J JGynecObst. 5: 038.
- Weissenbacher ER, Donders G, Unzeitig V, Halaska M, et al. (2012) A comparison of dequalinium chloride vaginal tablets (Fluomizin(R)) and clindamycin vaginal cream in the treatment of bacterial vaginosis: a single-blind, randomized clinical trial of efficacy and safety. Gynecol Obstet Invest. 73: 8-15.
- Breding K, Vikström I, Selbing A, Farnebäck M, Diagnosis of Bacterial vaginosis using a novel molecular real-time PCR test. J Women’s Health Gyn 7: 1-7.
- Rigby A (2002) Statistical methods in epidemiology. Towards an understanding of the kappa coefficient. DisabilRehabil 22: 339-344.
- Sobel J, Peipert JF, McGregor JA, Livengood C, et al. (2001) Efficacy of clindamycin vaginal ovule (3-day treatment) vs. clindamycin vaginal cream (7-day treatment) in bacterial vaginosis. Infect Dis Obstet Gynecol. 9: 9-15.
- McCormack WM, Covino JM, Thomason JL, Eschenbach DA, et al. (2001) Comparison of clindamycin phosphate vaginal cream with triple sulfonamide vaginal cream in the treatment of bacterial vaginosis. Sex Transm Dis. 28: 569-575.
- Swidsinski A, Mendling W, Loening-Baucke V, et al. (2008) An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 198: 97 e1-6.
- Machado A, Castro J, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Cerca N (2016) Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Frontiers in Microbiology 6: 1528.
- Mendling W, Palmeira-de-Oliveira A, Biber S, Prasauskas (2019) An update on the role of Atopobiumvaginae in bacterial vaginosis: what to consider when choosing a treatment? A mini-review. Arch Gynecol Obstet 300: 1–6.

Tables at a glance