Evaluation Phytochemical and Determination of Biological Activities of Different Extracts of the Leaves (Malus Pumila)
Received Date: March 03, 2023 Accepted Date: April 03, 2023 Published Date: April 06, 2023
doi: 10.17303/jmph.2023.2.101
Citation: Hafsia Bouzenna, Chiheb Soltani, Samira Jbahi, Mourad Raddaoui, Sondes Missaoui et al. (2023) Evaluation Phytochemical and Determination of Biological Activities of Different Extracts of the Leaves (Malus Pumila). J Med Plant Herbs 2:1-10
Abstract
The objective of this study was to evaluate the phytochemical of the extracts of the leaves of Malus pumila palnt and explore their antiradical and antimicrobial activities. Phytochemical screening revealed the presence of tannins, flavonoids, and polyphenols in the extracts prepared using chloroform, petroleum ether, and ethyl acetate solvents. Quantitative assays of polyphenols, total flavonoids, and tannins showed that the chloroform extract obtained using the Soxhlet method contained the highest content of polyphenols and flavonoids, respectively at (242.59±1.73 μg EAG/mg of dry extract) and (125.77±8.76 EQ/mg of extract), compared to the other extracts. All extracts demonstrated significant antiradical activity against the DPPH radical and strong reducing power (FRAP). Additionally, growth inhibitory effects were observed for most of the bacterial strains tested, including Escherichia coli, Staphylococcus, and Enterococcus faecalis, for all the extracts of Malus pumila. These results suggest that this plant has beneficial potential that can be explored in vivo for certain physiological functions.
Keywords: Malus Pumila; Polyphenols; Flavonoids; Tannins; Antioxidant Activity; DPPH; FRAP; Antimicrobial Activities
Introduction
Nature has been a source of medicine and herbal systems for thousands of years, and continues to play an essential role in primary health care around the world [1]. Medicinal plants are of primary interest to humans for their therapeutic potential, and have been used in traditional practices since antiquity [2]. They represent a source of bioactive compounds such as alkaloids, essential oils, tannins, saponins, phenolic compounds, and flavonoids [3]. In light of the need for phytotherapy, this study aimed to investigate the biological and antioxidant properties of apple leaves. The apple tree (Malus pumila) belongs to the Rosaceae family and has been shown to have antioxidant properties in numerous studies [4]. As a result, the apple tree has been found to reduce the risk of cardiovascular disease [5]. Apple leaves, which are often considered waste products, could potentially be a valuable raw material for the preparation of extracts or phenolic antioxidants [6]. There are several potential mechanisms of action for the bioactive compounds identified in apple leaves, which may contribute to their health benefits. The leaves contain phenolic compounds, such as flavonoids and phenolic acids, that can act as antioxidants and scavenge free radicals in the body, reducing oxidative stress and preventing cellular damage. Secondly, several studies have suggested that apple leaf extract may have a beneficial effect on glucose metabolism, reducing blood sugar levels and improving insulin sensitivity. This may be due to the presence of compounds such as phloridzin and quercetin. Phloridzin has been shown to reverse glucotoxicity and reduce blood glucose levels without increasing body weight, demonstrating its benefits in the prophylaxis and treatment of type 2 diabetes [7]. Finally, apple leaves contain compounds such as triterpenoids and catechins, which have been shown to have antimicrobial properties, potentially helping to prevent infections [8]. Overall, the bioactive compounds found in apple leaves may have a range of potential health benefits, contributing to the overall nutritional value of the plant.
Nevertheless, some studies have suggested that higher doses of apple leaf extracts may be toxic due to the presence of compounds such as phlorizin, sorbitol, and hydroxycinnamic acids. Long-term consumption of apple leaves or excessive doses of apple leaf extracts could lead to negative effects such as liver toxicity, kidney damage, and oxidative stress.
Nevertheless, some studies have suggested that higher doses of apple leaf extracts may be toxic due to the presence of compounds such as phlorizin, sorbitol, and hydroxycinnamic acids. Long-term consumption of apple leaves or excessive doses of apple leaf extracts could lead to negative effects such as liver toxicity, kidney damage, and oxidative stress.
The use of apple leaves has the potential to promote sustainable and environmentally friendly practices through the extraction of their bioactive compounds. This process can be achieved through the use of green technologies, such as supercritical fluid extraction, microwave-assisted extraction, and ultrasound-assisted extraction, which minimize the use of harmful chemicals and reduce the environmental impact of the extraction process. Furthermore, the bioactive compounds extracted from apple leaves have demonstrated potential health benefits and can be utilized in various industries, including pharmaceuticals, cosmetics, and food. This provides an alternative source of these compounds, which are traditionally derived from non-renewable sources or synthetic production methods [9].
The findings from the phytochemical evaluation and determination of biological activities of Malus pumila leaf extracts have broad practical applications in several fields. These include medicine, where the extracts of Malus pumila can be used to develop new drugs. In the food industry, they can also be used as natural food color additives. In agriculture, the extracts could be used as a natural pesticide to protect crops from pests and fungal infections.
Materials and Methods
Plant Material
The botanical name of the plant used in this study was Malus pumila. The leaves of the plant were collected from the Sidi Aich region of Gafsa, Tunisia between March and April 2021. The region is characterized by a longitude of 8.8E, latitude of 34.683N, altitude of 522 m, and an annual rainfall of 150 mm. After collection, the leaves were cleaned and allowed to dry for 15 days at room temperature (37°C) away from direct sunlight. Once dry, the leaves were crushed.
Preparation of Malus Pumila Extracts
The extracts were prepared using the following method
Soxhlet Extraction
Soxhlet extraction is a simple and convenient method that allows for the extraction cycle to be repeated infinitely with fresh solvent until the complete depletion of the solute in the raw material (Figure 1). The extraction was carried out in several cycles using 9 g of leaf powder in 250 ml of solvent (ethyl acetate, chloroform, and petroleum ether). The solvents used in the extraction process were classified according to their polarity. The extraction was carried out for 6 hours at temperatures between 30 and 600C. When the solution in the thimble became clear, it signified that the oil had been completely extracted, and the apparatus was switched off. The extraction process consisted of 7 cycles. After filtration, the extract was dried under heat and then scraped.
Yield Calculation
The yield is calculated as the ratio between the weight of the extract obtained and the weight of the dry plant matter, and is expressed as a percentage using the following formula. R (%) = Pb/Pa * 100
Where R is the yield in percentage, Pa is the weight of the extract obtained in grams, and Pb is the weight of the plant biomass in grams.
Phytochemical Tests
Qualitative Assays
This assay makes it possible to detect the different chemical families present in plants by coloring reactions.
Phenolic Compounds Test
The extract was dissolved in 3 mL of distilled water and 5 drops of FeCl3 were added to it. The development of the greenish color indicates the presence of phenols [10].
Flavonoid Test
The presence of flavonoids is confirmed by adding the extract to 100 μL of concentrated HCl and a few pieces of magnesium, resulting in the appearance of a red or orange color.
Tannin Test
The extract was tested for the presence of tannins using iron trichloride (FeCl3) 1% solution. Gallic tannins presence is indicated by a black-blue colour while catechin tannins presence is indicated by a greenish-blue colour.
Quantitative Assays
Assay of Total Polyphenols
The assay for total polyphenols was conducted following the method of Singleton et al. (1999) [11] using the Folin-Ciocalteu reagent. To begin, 50 μL of diluted plant extract was mixed with 1 mL of Folin-Ciocalteu reagent. After 4 minutes, 500 μL of 7.5 g/L sodium carbonate (Na2CO3) was added. The reaction mixture was then incubated for 30 minutes in the dark at room temperature, after which the absorbance was measured at 725 nm. The concentration of total polyphenols was determined using the calibration curve regression equation (y = 0.001x, R² = 0.9285) obtained with gallic acid as a positive control at concentrations ranging from 50-200 μg/mL and was expressed in micrograms of gallic acid equivalents per milligram of extract (μg EAG/mg of extract).
Dosage of Flavonoids
The quantification of flavonoids was carried out using an adapted method with aluminum trichloride and sod [12]. Briefly, 500 μL of the extract was added to a 5% sodium nitrite solution (150 μL) and 2 mL of distilled water. The tubes were incubated at room temperature for 6 min. Then, a 10% solution of aluminum chloride (75 μL) was added to the mixture, followed by 2 mL of 1 M sodium hydroxide. The solution was shaken well and incubated for 15 min, and the absorbance was determined at 510 nm. To quantify flavonoids, a calibration curve regression equation (y = 0.0006x, R² = 0.972) was prepared using quercetin solutions as a positive control at different concentrations (100, 250, 500, 750 µg/mL). The results are expressed in μg of quercetin equivalents per milligram of extract (μg EQ/mg of extract).
Dosage of Condensed Tannins
Condensed tannins were quantified using the vanillin method under acidic conditions. This method is based on the reaction between vanillin and condensed tannin units in the presence of acid, resulting in the formation of a colored complex that is measured at 500 nm [13]. To perform the assay, 200 μL of the sample or standard solution is added to 3 mL of a vanillin solution (4% in methanol) and 1.5 mL of concentrated hydrochloric acid, and incubated for 15 minutes. The concentration of condensed tannins is determined using a calibration regression equation (y = 0.0008x, R² = 0.9008) established with tannic acid (0-300 μg/mL) and expressed in micrograms of tannic acid equivalent per milligram of extract (μg TAE/mg of extract).
Antibacterial Activity
Test Microorganisms
The extracts were screened against three bacterialstrains: Escherichia coli (ATCC25922), Staphylococcus aureus(ATCC29213), and Enterococcus faecalis (ATCC29212),which were identified and obtained from the hospital in the Gafsa region. Imipenem, Linezolid, and Ticarcillin were used as reference antibiotics.
Agar-well Diffusion Method
The antibacterial activity of the extracts was evaluated using the agar well-diffusion method. The bacterial strains were first cultured on Muller Hinton medium to obtain isolated colonies. After 24 hours of incubation at 37°C, four or five colonies were isolated and transferred into a tube containing distilled water, and the turbidity was adjusted to 0.5 McFarland standard. Using a sterile Pasteur pipette, four wells of 6 millimeters in diameter were made, well-spaced from each other. Then, 20 μl of each extract concentration (C1: 10 mg/ml, C2: 10 mg/ml) was poured into the wells. The Petri dishes were kept for 1 hour and incubated at 37°C for 24 hours. The results were expressed as the zone of growth inhibition surrounding the wells.
Statistical Analysis
All biochemical and bioactivity experiments were carried out three times, and for each experiment, three biological replicates were performed. All data were expressed as the mean
± SE (standard error of the mean). Significance level was determined (p<0.05) and significant difference was separated using Duncan’s Multiple Range Test.
Results and Discussion
Yield of extraction
The most important yield obtained in this study, in the extracts of the leaves of Malus pumila, was that of ethyl acetate (EAS), which is around 6.3% than other extract petroleum and chloroformed which were 3.51% and 3.21% respectively. Our results differ from other studies [14] which reported that the yield of ethanolic apple leaf extract is equal to 13.6% (Figure 2).
This variability in yields can be attributed not only to the geographical origin of the plant, but also to other factors such as the time of harvest and/or the way of conservation. Our results also confirm the studies carried out by Kim et al. [15], which showed that the method used (the choice of solvents), the conditions under which the extraction is carried out (hot or cold) and the extraction time, can vary the extraction yields, affecting the total content into secondary metabolites. The biological activities explored by these metabolites can also be varied.
Phytochemical Screening
Table 1 presents the results of phytochemical screening conducted on the leaves of Malus pumila, which revealed that all extracts were rich in bioactive compounds such as flavonoids, phenolic compounds, and tannins.
Total Phenol, Flavonois and Tannins Content
The results obtained were illustrated in figure (3) and showed the chloroform extract obtained by the Soxhlet method had the highest levels of polyphenols and flavonoids compared to the other extracts. The levels of polyphenols and flavonoids in this extract were 242.59 ± 1.73 μg EAG/mg of dry extract and 125.77 ± 8.76 EQ/mg of extract, respectively. These levels are higher than those reported in other studies which estimated the levels of polyphenols and flavonoids to be 163.35 ± 4.36 µg EAG/mg and 45.02 ± 0.90 µg EQ/mg, respectively [1].
Anti-Radical Activity
Anti-Radical Activity Against the DPPH Radical
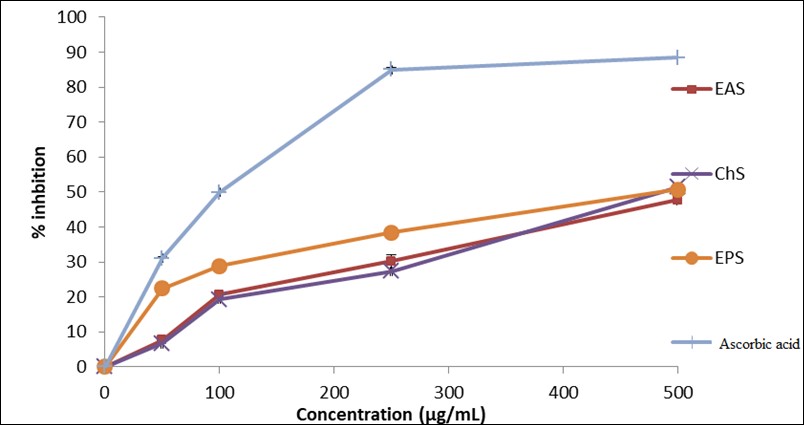
Based on the results obtained, it can be inferred that the anti-radical activity against the DPPH radical studied in vitro is dose-dependent, as shown in Figure 4. The increase in extract concentration correlates with an increase in anti-DPPH activity. These results were quantified using the IC50 parameter, which is the concentration of the substrate that leads to a 50% reduction in activity. Lower IC50 values indicate more potent anti-radical activity. In this study, the extract with the lowest IC50 value exhibited the most powerful anti-radical activity, and the concentration of the extracts yielded a value of CI50= 500 μg/mL. This reflects a potent antioxidant activity compared to the other extracts. These findings support the results of a previous study by Pranas et al [15], which reported that Malus extracts showed significant antioxidant potential.
Anti-Radical Activity Against the DPPH Radical
The reducing power of apple leaf extract and BHT standard were represented in the figure 5. The results showed that the reducing power increased with the concentration. Indeed, the extract (EPS) has the highest reducing power. The work of Pranas et al [15] reinforced our deductions reporting that the richness of apple tree extract in several bioactive compounds stimulates its antioxidant capacities to neutralize the free radicals generated.
In this study, the antibacterial activity of Malus pumila extract was evaluated using the well diffusion method on three bacterial strains: Staphylococcus aureus, Escherichia coli, and Enterococcus faecalis. IMIPENEM, LINEZOLID, and TICARCILLIN were used as reference antibiotics for E. coli, E. faecalis, and S. aureus, respectively, while DMSO served as a negative control. The results, presented in Table 2, showed that the different strains exhibited sensitivity to the various extracts, with inhibition zones ranging from 22 to 34 mm for the reference antibiotics. The results differed from those found by Sowa et al. [17], who reported that Malus domestica leaf extracts only exhibited growth inhibitory activity against two Gram-positive bacterial strains, Staphylococcus aureus and Enterococcus faecalis, and against the Candida glabrata fungus strain. The antimicrobial effect of a substance depends on both its concentration and the sensitivity of the microorganism to the substance. In 2016, Sowa et al. demonstrated that Malus domestica extracts contain a sufficient concentration of phenols to exhibit antibacterial activity.
Antibacterial activity
Conclusion
The bioactive compounds identified in apple leaves may have a range of potential health benefits, including anti-inflammatory, antioxidant, anti-diabetic, and anti-obesity effects. It has been reported that the antioxidant activity of apples is highly correlated with the total phenolic content, and epicatechin and procyanidin are significant contributors to the antioxidant activity of individual compounds. The process of investigating the pharmacokinetics and pharmacodynamics of active compounds involves studying how the body interacts with the drug, including its absorption, distribution, metabolism, and excretion. This helps to understand the drug's mechanism of action within the body and informs its potential for drug development. Through this research, drug developers can identify potential therapeutic benefits and side effects, evaluate the appropriate dosage and formulation, and assess drug efficacy and safety.
Declaration of Conflicting Interests
The authors declare that there are no conflicts of interest
Orcid
Hafsia Bouzenna https://orcid.org/0000-0003-00 40-6417
- Pazyar N, Yaghoobi R, Rafiee E, Mehrabian A, Feily A (2014) “Skin wound healing and phytomedicine: A review”, Skin PharmacolPhysiol 27: 303-10.
- Moglad EH, Alhassan MS, Abdalkareem EA, Abdalla AN, Kuse M “Ethyl acetate fraction of Solanumnigrum L Cytotoxicity, induction of apoptosis, cell cycle in breast cancer cells, and gas chromatography-mass spectrometry analysis”, Asian J. Pharm 13: 246-51.
- Ibrahim NI5, Wong SK, Mohamed IN, Mohamed N, Chin KY et al. (2018) “Wound healing properties of selected natural products”, International journal of environmental research and public health 15: 2360.
- Wojdyło A, Oszmiański J (2020) “Antioxidant activity modulated by polyphenol contents in apple and leaves during fruit development and ripening”, Antioxidants 9: 567.
- Bonarska-Kujawa D, Cyboran S, Oszmiański J, Kleszczyńska H (2011) “Extraits de feuilles de pommier et de fruits comme antioxydants efficaces J. Med. Plants Res 5: 2339-47.
- Ceymann M, Arrigoni E, Schärer H, Bozzi, Nising A, Hurrell RF (2012) “Identification de pommes riches en flavan-3-ols et acides phénoliques bénéfiques pour la santé en mesurant le profil polyphénolique". Journal de la composition et de l'analyse des aliments 26: 128-35.
- Liaudanskas M, Viškelis P, Raudonis R, Kviklys D, Uselis N et al. (2014) Phenolic composition and antioxidant activity of Malus domestica leaves. The Scientific World Journal.
- Graziani G, D Argenio G, Tuccillo C, Logurrcio C, Ritieni A et al. (2005) Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut 54: 193-200.
- Caliceti C, Malaguti M, Marracino L, Barbalace MC, Rizzo P et al. (2022) Agri-Food Waste from Apple, Pear, and Sugar Beet as a Source of Protective Bioactive Molecules for Endothelial Dysfunction and Its Major Complications. Antioxidants 11: 1786.
- Rosine C, Momo D (2009) “Évaluation de l’activité antidermatophytique des extraits au méthanol et fractions d’Acalyphamma hirtum (melastomatacees)’’ Université de Dschang -Master en biochimie clinique et pharmacologie.
- Singleton VL, Rossi JA (1999) “Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. American”. Journal of Enology and Viticulture 16: 144-58.
- . Dewanto V, Wu X, Adom KK, Liu RH (2002) “Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity”, Journal of Agricultural and Food Chemistry 50: 3010-4.
- Makkar HPS, Becker K (1993) “Vanillin-HCl method for condensed tannins: effect of organic solvents used for extractions of tannins”, Journal of Chemical Ecology 19: 613-21.
- Yanzhen L, Yang D, Xiaoyan Q, Huantong W, Yanjun H et al. (2019) “Comprehensive evaluation of effective polyphenols in apple leaves and their combinatory antioxidant and neuroprotective activities”, Industrial Crops and Products 129: 242-52.
- Kim JH (2017) Extraction time and temperature affect the extraction efficiencies of coumarin and phenylpropanoids from Cinnamomum cassia bark using a microwave-assisted extraction method, Journal of Chromatography B 1063: 196-203.
- Pranas V, Raimondas R, Darius K, Norbertas U, Valdimaras J (2014) Composition phénolique et activité antioxydante des feuilles de Malus domestica 306217.
- Sowa A, Zgórka G, Szykuła A, Franiczek R, Żbikowska B et al. (2016) Analyse de composés polyphénoliques dans des extraits de feuilles de certains cultivars de Malus domestica analyse antiradicalaire et antimicrobienne de ces extraits. BioMed Research International 5: 1-12.

Tables at a glance
Figures at a glance