Seroprevalence and Allied Risk factors of Small Ruminants Bluetongue Disease virus in Bedeno and Gumbi Bordode districts of Hararghe zone, Oromia National Regional state, Eastern Ethiopia
Received Date: April 17, 2023 Accepted Date: May 17, 2023 Published Date: May 20, 2023
doi: 10.17303/javm.2023.2.102
Citation: Ashebir Abebe, Abdela Bulbula, Yitbarek Getachew, Kemal Emyu, Kebede Debebe (2023) Seroprevalence and Allied Risk factors of Small Ruminants Bluetongue Disease virus in Bedeno and Gumbi Bordode districts of Hararghe zone, Oromia National Regional state, Eastern Ethiopia. J Anim Biol Vet 2: 1-8
Abstract
Bluetongue, the pathogenic disease of sheep, goat, cattle, camels, llamas, deer and antelopes is caused by the virus genus of Orbvirus, which is classified under the family Reoviridae. A Cross-sectional study was conducted to determine the seroprevalence and allied risk factors for bluetongue virus infection in Bedeno and Gumbi Bordode districts of Hararghe zone. Total of 384 blood samples were analyzed using cELISA for detection of antibody, which is specific to the blue tongue virus. Accordingly, 48.4% (n = 198) serum samples were positive on cELISA. Among the examined animal, bluetongue virus was detected in 66.7% in sheep and 44.4% in goats. The seroprevalence of bluetongue is significantly associated with the species ovine (P<0.05). Hence, sheep had 2.5 times higher odds to be positive for bluetongue on cELISA. Univariate analysis of potential factors indicated that species, sex, age of the animals and agro-ecology factors are significantly allied (P<0.05%) with seroprevalence of the BTV. However, agro-ecological factor was determined as predicting variable for BTV infection on analysis, whereby, the odds of infection at lower land is 23.4 times higher compared to highlands. In conclusion, the present study revealed the presence of bluetongue infection among sheep and goats of Hararghe zones with higher detection in lowland areas. Therefore, further studies are suggested to determine the bluetongue serotypes that are circulating in sheep and goats in Ethiopia and vector studies in order to devices control strategy feasible to be implemented at different agro-ecological conditions.
Keywords: Bedeno; Bluetongue virus; Gumbi Bordode; cELISA; Seroprevalence
Introduction
Ethiopia is commonly known African country with large number of livestock population. As the evidence of CSA. (2013) indicated, the country is populated by 25.5 million sheep and 24.06 million goats.
Despite having huge number of livestock population, there is no more evidence of Bluetongue disease virus and also, there is less information about the transmitting vector in the country other than the recent study conducted in the western part of Amhara region, which identified 34.1% in sheep [1] and 46.67% seroprevalence in sheep of different agro-ecological areas of central Ethiopia [2].
The Bluetongue, the pathogenic disease of sheep, goat, cattle, camels, llamas, deer and antelopes is caused by the virus genus of Orbvirus, which is classified under the family of Reoviridae [3]. It has about 29 strains, which are known for their causing the infection in susceptible hosts [4]. The presence of different virus species of Bluetongue disease has been a great role in determining the severity of the disease in animals [5].
The disease highly known by its effect of causing great economic losses by killing of infected animals, decreasing of body weight, low reproduction capacity directly, and limiting of export animals and animal products by indirectly manner [6].
The transmission of the virus is by biting strains of Culicoides, which is mainly the C. imicola in different parts of Africa and southern Europe [7]. The disease is commonly known by harming the Ovine, fine-wool and mutton breeds. Although, it can cause infection in Bovine, caprine and susceptible wild ruminants [8].
In history, the name “Bluetongue disease “was originated from its capability in causing cell injury and necrosis which finally resulted to edema, vascular thrombosis and hemorrhage and leads in cyanotic(bluetongue) [9]. According to the [10], the disease was discovered in South Africa’s Cape Colony after the introduction of the so called merino sheep breed during the end of 18th century and then ultimately to another African country, Europe, the Middle East, Indian subcontinent, America and Asia in exception of Antarctica.
The drooling of saliva, oral lesion, facial edema, depression, anorexia, fever, nasal discharge and the faintness of muscle are considered as clinical signs of Bluetongue irus in wild ruminant animals and in ovine [3].
In diagnosis of Bluetongue disease virus, the advanced laboratory tests such as; the isolation of the virus by using an embryonated egg of chicken, indirect Enzyme linked immuno-sorbent assay (I- ELISA) and competitive enzyme-linked immunosorbent assay (c-ELISA) for antibody detection, agar gel immune diffusion test (AGID) and polymerase chain reaction (PCR) are very important [11].
The present status of bluetongue disease virus in Ethiopia, and its transmitting vectors are less investigated. This could be due to less attention to this virus as another commonly known more small ruminant diseases with related clinical signs. Despite this, only a few studies have been conducted in identifying the antibodies against the bluetongue virus in Ethiopian small ruminants. For instance, there is absence of published evidence in eastern part of Ethiopia. Thus, the present study objective was generally; to determine the Seroprevalence of bluetongue disease virus, and to identify the allied risk factors with the bluetongue disease virus in small ruminants in Gumbi Bordode and Bedeno district, Eastern Ethiopia.
Materials and Methods
Study areas
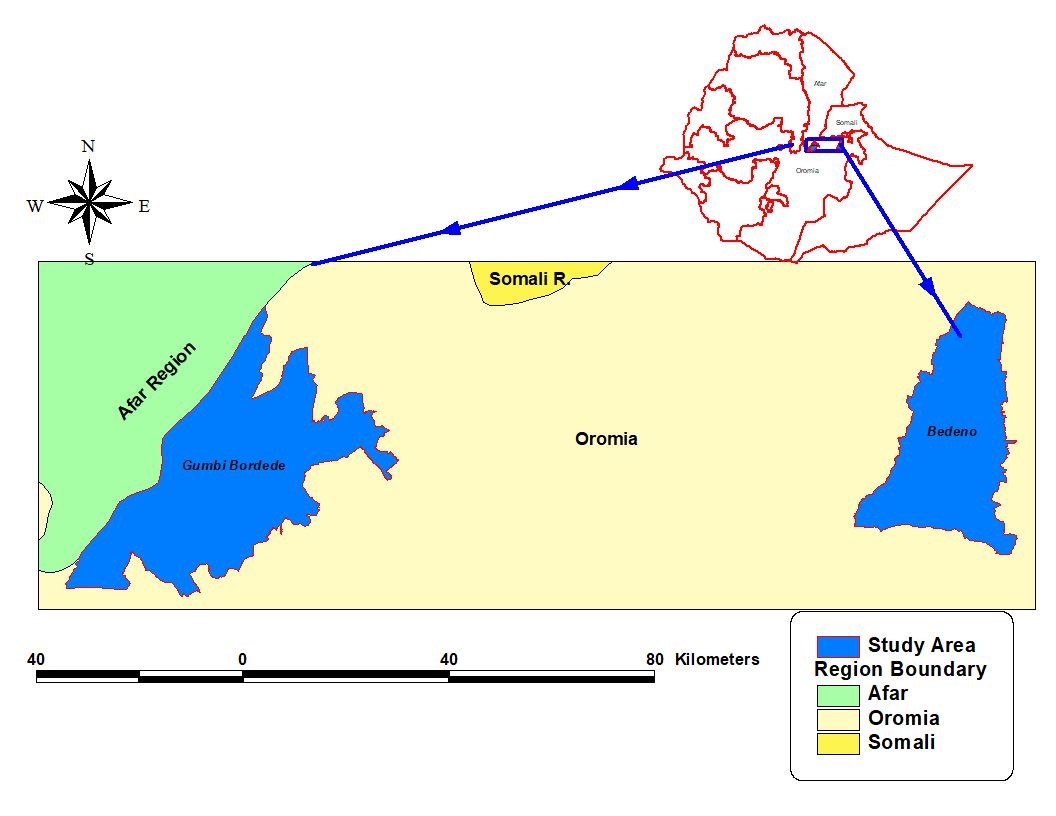
Study was conducted in selected two districts of Oromia region Bedeno and Gumbi Bordode district parts of East and West Hararghe zone respectively from April/2021 to January/2022. The two district included in this studies are located and found in different agro ecological zone.
Gumbi Bordode district: is located 251 km southeast of the capital Addis Ababa Gumbi Bordode district which was previously part of Mieso district is bordered in the west and North West with Afar regional state, in the south with Anchar District, in the southeast with Guba Koricha district and in the east with Mieso district. The most of this district area are located in Rift valley and due to this,the western area of the district experience high daily temperature throughout the year.
Bedeno district: it is Located in East Hararghe zone, bordered in the west with Deder and Goro muti, in the north with Meta and Kersa district, in the south with Gola oda district and in the East with Grawa district. Bedeno altitude ranges from 1200-3100 MASL (MeterAbove Sea Level) with highest point peak at Aneya Genem (Ethiopia Mapping Agency Topographic Map 1; 50000). Bedeno Kebeles the lively hood of farmers depend on cash crop production area of the land is mainly covered with crop land and cat plantation, and communal grazing land is scares and livestock in highland area are graze on privet small area around the vicinity of the farm or fed at back yard. Gumbi Bordode disrict which is located in the lowland area of the Great Rift Valley animals graze free, and feed availability during dry season is scarce specially during sudy period in the of March to December /2021, animals were suffering due to shortage of feeds and scarcity of water, at a time of sampling there was on going government help supplying grass feed for livestock at a time.
Study population
The study population includes sheep and goats, under extensive production system in Bedeno and Bordode districts. The study included sheep and goats of all sexes and ages that were not vaccinated for PPR. Dentation equations were used to estimate the age of the animals. Animals were split into two groups based on their age: young (less than or equal to one-year-old) and adult (less than two-year-old)
Study design
A cross-sectional study was carried out during this study period. In both districts, a blood samples were randomly collected from small ruminants (goats and sheep).
Sample size determination
The optimum sample size to estimate the seroprevalence of bluetongue was computed using the formula provided by Thrusfield (2005) with a 95 percent confidence interval and a 5% absolute precisionas indicated below:
N=1.96²× P exp(1- P exp)/d²
Where, N = sample size, P exp = expected prevalence,And d = desired absolute precision
Because there had been no previous research work in the study area, the projected prevalence rate was taken as 50 percent, and thus a total of 384 goats and sheep serum samples were collected from Bedeno and Gumbi Bordode districts for the serological test.
Sampling procedure
About 5 ml of blood was collected aseptically from the jugular vein of each sheep and goat using plain serum vacutainer tubes and needles. The blood is allowed to clot over night at room temperature and 2ml serum were then decant in to cryovials and each cryovials is labeled with permanent water resistant marker and stored in Ice box containing cooled ice pack; and related data of individually sampled animals such as; age, species sex and other information is written on sample submission format as well as entered in to Ms-excel sheet GPS coordinate Latitude, Longitude and Altitude was collected at the sampling site.
Laboratory test
TThe collected Serum samples were examined for the presence of BTV group specific antibodies, by using a competitive enzyme-linked immunosorbent assay (c-ELISA) ID Screen® Bluetongue Competition-Idvet with Proven specificity and sensitivity, and widespread use in recent outbreaks.
Data management and analysis
Data collected were entered in to Microsoft Excel spreadsheet and descriptive statistic was done using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Chi-square test was applied to analyze the association between sero-prevalence status among age, sex, breed and specie P-values less than or equal to 0.05 were considered to be significant.
Results
Seroprevalence of bluetongue infection
Out of 384 sera tested by c-ELISA, 186 (48.4%) were found positive against BTV-specific antibodies. The seroprevalence of the Bluetongue disease virus (BDV) among the sheep and goat population was 66.7% (n=46) and 44 %( n=140) respectively (Table 1).
The Allied risk factors
Host and agro-ecological factors that potentially affect the occurrence and transmission of the bluetongue virus were considered for chi-square and logistic regression analysis. Accordingly, species of animals, sex, age and agroecology had a significant association (p< 0.05) with the seroprevalance of bluetongue virus on Chi-square test (Table 2).
It was found that the sheep had 2.5 time more odds to be sero-reactive than goats. Females are two times more infected than male animals. Significantly higher adult animals were seropositive than young animals (OR: 77[4.48, 11.1]). Animals of lowland were 13.4 more chance to be infected by bluetongue virus compared to those animals in high land areas.
Discussion
The shoats (sheep and goats) were identified highly as prevalent (48.4%) against the antibody of Bluetongue virus (BTV) during the present study. This is similar with the study results of [2]. which was detected 46.67% in sheep from central Ethiopia, and that of [12] in Wolaiyta Zone of southern Ethiopia, 41.17% in small ruminants and 45.7% identified in small ruminant in India, by [12]. It is differing from the findings of [7] in south western Ethiopia in small ruminants, which was lower as of 30.6%, 6.96% from small ruminants (13.7% in goats and 5.70% in sheep) in Algeria [14], in small ruminants in Turkey (29.5%) [15] and sheep in Iran (37.7%) [16].
Hence, the risk factors such as Agro-ecology has huge impact on sero-prevalence of BTV antibodies. Accordingly, the greater prevalence of BTV (82.25%) was identified in lowland area of Gumbi Bordode district. This indicated that, lower altitudes are favorable for propagation of arthropod (Culicoides) as a result of warmer condition in this areas [2], which is the main transmitter for the virus. This is in lined with the study findings by [7]. Conversely, the smaller number of seroprevalence, which was 15.63% of examined animals was found from the highland areas of Bedeno district. This is due to the weather which is not very well for Culicoides breeding [7,17].
During this study, the bigger seroprevalence of BTV antibody was detected in ovine (66.7%) than Caprine (44.4%). This result is in relation to the finding by [2] and differ from that of [7].
In addition, the seroprevalence of BLV is more identified in aged (Adult) than younger small ruminants. This shows that, the infection can be advanced in older animals than younger ones as adult shoats can move freely to grazing areas and can easily contract the virus from different areas. Also, younger animal is commonly managed in save places [18].
On another hand, both of the female and male animals were prevalent to BTV. However, it is not necessary for comparing their results because of unequal number of them during study period.
The clinical case of bluetongue was not observed among animals sampled during clinical examination and so far no cases have been reported in Ethiopia. The absence of clinical disease suggests that indigenous breed of sheep andgoats have a high degree of innate immunity as elaborated by [19].
Conclusions
The present study provided serological evidence for presence and circulation of bluetongue virus among sheep and goat population in Hararghe zone. The seroprevalence of the virus was 48.4%. Univarate analysis identified species, age, sex and agro-climate conditions variables as potential epidemiological factors affecting the prevalence of bluetongue virus in the study population. However, agro-ecological factor was determined as predicting variable for BTV infection where significantly higher infection was noted in low land compared to higher land of study area. Based on the findings further studies are suggested to determine the bluetongue serotypes that are circulating in small ruminants in Ethiopia, the economic impact of the disease and vector studies in order to devices control strategy feasible to be implemented at different agro-ecological conditions.
Acknowledgment
I would like to acknowledge Bedeno and Gumbi Bordode veterinary personnel’s and District agriculture office,Hirna laboratory professionals Dr Kifele and Dr Biniyam. Our special gratitude to Animal health institute (AHI) for their great help by everything needed from the beginning to accomplishment of the study.
Conflict of the Interest
No conflict of interest observed between Authors.
- Gulima D (2009) Seroepidemiological study of bluetongue in indigenous sheep in selected districts of Amhara National Regional State, north western Ethiopia. Ethiop Vet J 13: 1-15.
- Woldemeskel M, Tilahun G, Tibbo M. Potgieter LN (2000) Prevalence of bluetongue virus antibodies in sheep in central Ethiopia. DtschTierarztl Wochenschr 107: 408-10.
- Ma J, Zhang X, Zheng W, Xu Y, Zhu X et al. (2017) Seroprevalence and Risk Factors of Bluetongue Virus Infection in Tibetan Sheep and Yaks in Tibetan Plateau, China.
- Legisa D, Gonzalez F, De Stefano G, Pereda A, Santos MD (2013) Phylogenetic analysis of bluetongue virus serotype 4 field isolates from Argentina. J Gen Virol 94: 652-62.
- James NM, Edward JD (2011) Fenner’s Veterinary Virology. 4th ed. Elsevier Inc 2: 283-5
- Aradaib IE, Mohamed ME, Abdalla TM et al. (2005) Serogrouping of United States and some African serotypes of bluetongue virus using RT-PCR. Vet Microbiol 111: 145-50.
- Abera T, Bitew M, Gebre D, Mamo Y, Deneke Y, Nandi S (2018). Bluetongue disease in small ruminants in south western Ethiopia: Cross-sectional sero-epidemiological study. BMC Research Notes, 11.
- Merck vet manual https://www.merc kvetmanual.-com/generalized-conditions/bluetongue/overview-of-bluetongue
- Murphy FA, EPJ Gibbs, MC Horzinek, MJ Studdert (2011) Reoviridae Diseases Caused by Members of the Genus Orbivirus, Bluetongue. In: Veterinary Virology, 3rd Edn. Elsevier, San Diego, California USA 398-400.
- Hoffmann BB, Bauer C, Bauer HJ, Bätza M, Beer PH, Clausen A Liebisch (2009) Monitoring ofputative vectors of bluetongue virus serotype 8, Germany. Emerg Infect Dis 15: 1481-4.
- OIE (Organization International des Epizooties).Manual of diagnostic tests and vaccines for terrestrial animals contagious caprine pleuropneumonia; 2014.
- Yilma M, Mekonnen M (2015) Competitive enzyme linked immuno-sorbent assay (c-ELISA) based sero-prevalence of bluetongue virus (BTV) on small ruminants in selected areas of Wolaita, Southern Ethiopia. J Virol Mycol 4: 148.
- Sreenivasulu D, Subba Rao MV, Reddy YN (2004) Overview of bluetongue disease, viruses, vectors, surveillance and unique features: The Indian subcontinent and adjacent regions. Vet Ital 40: 73-7.
- Moustafa K, Pam DL, Meriem HB (2016) Sero-epidemiology of bluetongue in Algerian ruminants. African J Biotechnol 15: 868-71.
- Gur S (2008) A serologic investigation of blue tongue virus (BTV) in cattle, sheep and gazella subgutturosa subgutturosa in southeastern Turkey. Trop Anim Health Prod 40: 217-21.
- Shoorijeh SJ, Ramin AG, Maclachlan NJ, Osburn BI, Tamadon A, Behzadi MA et al. (2010) High seroprevalence of bluetongue virus infectionin sheep flocks in West Azerbaijan,Iran. Comp Immuno Micro Inf Dis 243-7.
- Wilson AJ, Mellor PS (2009) Bluetongue in Europe:past, present and future. Philos Trans R Soc B 364: 2669-81.
- Jimenez-Clavero MÁ (2012) Animal viral diseases and global change: bluetongue and West Nile fever as paradigms. Front Genet 3: 105.
- Haresnape JM, Taylor WP, Lungu SAM (1998) The epidemiology of bluetongue in Malawi. Epidemiology and Infection 100: 493-9
- CSA. Federal Democratic Republic of Ethiopia central statistical agency agricultural sample survey, vol II Report on livestock and livestock Characteristics 15-25.
- Du-Toit RM (1944) The transmission of bluetongue and horse sickness by Culicoides. J. Vet. Anim. Ind 19: 7-16.
- Ghanem YM, Fayed AA, Saad AA, Abdelkader AH (2009) Serological survey on bluetongue virus infection in camels at two camel-rearing regions of Somalil-and. Assiut.Vet. Med. J 56: 77-87.
- Hammami S (2004) North Africa: a regional overview of bluetongue virus, vec-tors, surveillance and unique features. Vet Ital 40: 43-6.
- Maclachlan NJ (2011) Bluetongue: history, global epidemiology, and pathogenesis. Prev Vet Med 102: 107-11.
- OIE (World Organisation for Animal Health) (2020) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals.OIE, Paris, France.
- Toye PG, Batten CA, Kiara H, Henstock MR, Edwards L, Thumbi S et al. (2013) Bluetongue and epizootic haemorrhagic disease virus in local breeds of cattle in Kenya. Res Vet Sci 94: 769-73.

Tables at a glance
Figures at a glance