A Novel Treatment Approach for Women with Chronic Pelvic Pain Syndrome leading to Increased Pelvic Functionality
Received Date: August 13, 2020 Accepted Date: September 10, 2020 Published Date: September 12, 2020
doi: 10.17303/jwhg.2020.7.402
Citation:Aida Mustafa (2020) A Novel Treatment Approach for Women with Chronic Pelvic Pain Syndrome leading to Increased Pelvic Functionality. J Womens Health Gyn 7: 1-10.
Abstract
Background: This study was performed to evaluate the effectiveness of treatment of women with Chronic Pelvic Pain Syndrome (CPPS) using a combination of external ultrasound-guided trigger point injections to the pelvic floor musculature with peripheral nerve hydrodissection.
Methods: A retrospective study of 73 women with CPPS who were treated with external ultrasound-guided trigger point injections to the pelvic floor musculature with pelvic peripheral nerve hydrodissection once a week for six weeks in an outpatient setting. Pelvic pain intensity as measured pretreatment and post treatment using the Visual Analogue Scale and Functional Pelvic Pain Scale. Categories of function evaluated were bladder, bowel, intercourse, walking, sleeping, working, running, and lifting.
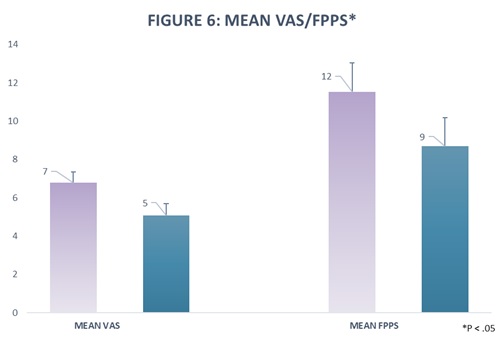
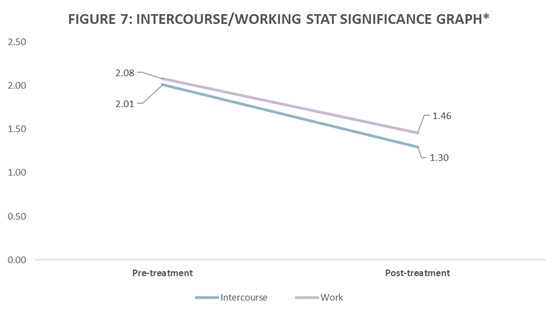
Results: Pretreatment, the mean VAS score was 6.8 (Standard deviation [SD] 2.38); P < .05, 95% confidence interval (CI) 6.25 to 7.35. Post treatment, the mean VAS score was 5.08, (SD 2.67); P < .05, 95% confidence interval (CI) 4.46 to 5.70. The mean total FPPS score before treatment was 11.53 (SD6.50); P < .05, 95% confidence interval (CI) 10.02 to 13.03. Post treatment, the mean FPPS score was 8.69, (SD 6.38); P < .05, 95% confidence interval (CI) 7.21 to 10.17. Analysis of the subcategories within the FPPS indicated that the improvement was statistically significant in the categories of intercourse and working. In the category of intercourse, the mean change in score after treatment was .72. Pretreatment, the mean was 2.01 (P < .05, 95% CI1.63-2.40). Post treatment, the mean was 1.29 (P < .05, 95% CI.96-1.63). In the category of work, the mean change in score after treatment was .62. Pretreatment, the mean was 2.08 (P < .05, 95% CI1.73-2.42). Post treatment, the mean was 1.46 (P < .05, 95% CI1.18-1.74).
Conclusion: Analysis suggests that the treatment was effective at ameliorating pain in women with CPPS. It showed promise in improving overall pelvic function in women with CPPS, specifically in the categories of intercourse and working.
Keywords: Pelvic Pain; Chronic Pelvic Pain Syndrome; Endometriosis; Central Sensitization; Neuropathic Pain; Neurogenic Inflammation; Trigger Points; Myofascial Pain.
Introduction
Chronic Pelvic Pain Syndrome (CPPS) is a complex, multi-faceted disease complex. CPPS is defined as pain associated with any pelvic structure that persists or recurs for at least 6 months and is not the direct result of a single, obvious, local pathology [1]. CPPS is one of the most prevalent gynecologic conditions affecting over nine million women in the United States [2]. It involves a constellation of symptoms arising from various organ systems within the pelvis [3], making diagnosis and treatment difficult. Associated dysfunction occurs in the gastrointestinal, urogynecology, and neuro-musculoskeletal systems, and often presents with psychosocial consequences [1]. More than one etiology is involved in over 50% of patients [4], though commonly implicated conditions include endometriosis, irritable bowel syndrome (IBS), interstitial cystitis, as well as neural and musculoskeletal pathologies [5].
The pathophysiology of CPPS is multifactorial, however, important underlying themes include myofascial pain, peripheral sensitization, and central sensitization [2,3]. Peripheral sensitization arises from the abnormal firing of peripheral nociceptors, sometimes due to a lesion within, or myofascial tension impinging upon, a nerve or group of nerves [6]. In peripheral sensitization, a repetitive insult creates neurogenic inflammation which reduces the activation threshold and intensifies the response of a nerve, causing hyperalgesia, increased reactivity of the peripheral nerve to noxious stimuli [3]. Central sensitization, common among chronic pain conditions, involves a maladaptive response to continuous pain signals that result in decreased action of inhibitory pathways and/or increased action of amplification pathways, leading to the magnification of the original insult. Continuous pain signals from any part of the pelvic anatomy can lead to central pain amplification and viscerosomatic cross-sensitization, producing hyperalgesia in neighboring, healthy structures [3].
Myofascial pain, characterized by muscle and connective tissue tenderness with localized and referred pain, is highly prevalent in those with CPPS [7]. Trigger points, a feature of myofascial pain, are palpable, taut areas of muscle which can render the muscle incapable of proper contraction and relaxation. They are painful to compression, may cause referred pain [8], and are associated with central sensitization [5]. Spasms, another feature of myofascial pain, are involuntary motor responses that constantly stimulate pain receptors [8]. They contribute to neurogenic inflammation and peripheral sensitization via nerve compression and neural ischemia [9]. Additionally, long-term, pain-related posturing overloads the pelvic muscles, ligaments, and joints, changing the pelvic floor structure, thereby exacerbating pain and dysfunction [8].
This multifaceted nature of CPPS makes it an extremely difficult condition to treat. Most available treatments address a solitary anatomic organ system. Response to treatment is often poor, with pain recurrence common [2]. Traditional treatment options for CPPS can be divided into hormonal, oral medications, and surgical [10,11]. Evidence-based treatment options remain limited despite the rising need [4]. The objective of this study is to determine the effectiveness of a non-operative neuromusculoskeletal protocol in addition to the traditional approaches for CPPS. The effectiveness of our protocol has been studied for a small number of patients with CPPS and pathology confirmed endometriosis [12]. The current study was conducted to provide further evidence for the use of our protocol with a larger sample size of women with CPPS of various etiologies.
Materials and Methods
Participants
Participants were 73 female patients between the ages of 19 to 74 years old who presented to an outpatient pelvic rehabilitation private practice and were diagnosed with CPPS. Patient demographics can be seen in Table 1. All participants underwent pretreatment evaluations with a detailed history and physical examination, including an internal pelvic floor examination performed by one of four physiatrists.
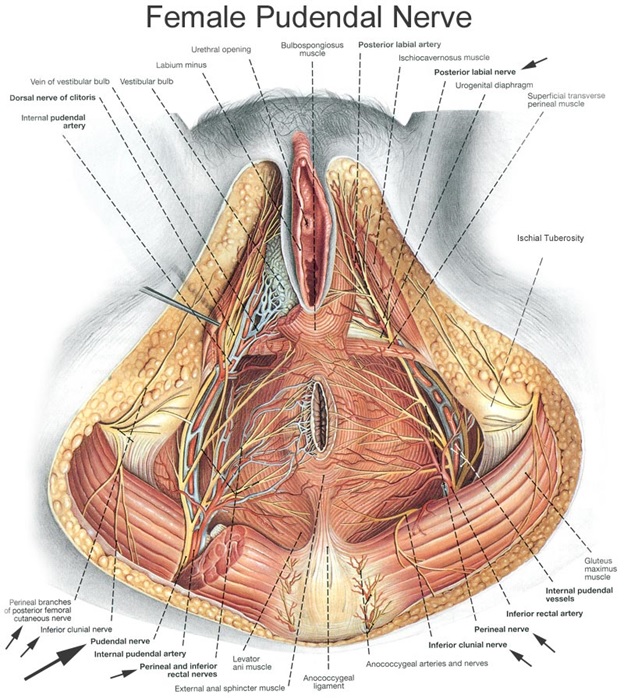
The internal exam included palpation of the levator ani sling to determine muscle strength, muscle tone, and the presence of trigger points. Trigger points are palpable, taut bands within muscles that are tender to palpation, and have a referred pain pattern. Pelvic floor trigger points often produce referred pain to the lower abdomen, medial thigh, buttocks, and perineum. The pudendal nerve was assessed with palpation over Alcock's Canal and the ischial spines to check for tenderness or a tingling sensation known as Tinel's sign. In addition, allodynia was noted at the posterior femoral cutaneous nerve between the quadratus femoris and the obturator internus.
The inclusion criteria included a history of CPPS of greater than 6 months duration and completion of at least 6 weeks of pelvic floor physical therapy. The exclusion criteria included malignancy and active pregnancy. The medications tried, relevant diagnoses, and past medical history, as well as the prior surgeries of patients, are displayed in Figures 1, 2, and 3, respectively.
Procedures
A retrospective chart review was done upon an institutional review board (IRB) approval (IRB# 17-0761). The protocol used for the intervention was developed for patients with CPPS who failed to progress after six weeks of pelvic floor physical therapy.
The protocol includes external ultrasound-guided trigger point injections targeting the pelvic floor musculature. Once a week for six weeks, the iliococcygeus, pubococcygeus, or puborectalis were injected unilaterally, alternating right and left sides throughout the protocol. With the patient lying in the prone position, a flexible, 6-inch, 27-gauge needle was used to inject the targeted muscle from the subgluteal posterior approach, using an aseptic technique under ultrasound guidance. Patients concomitantly underwent ultrasound-guided, peripheral nerve hydrodissection of the pudendal nerve at Alcock's Canal while in the prone position. The patient was then flipped to the supine position and underwent ultrasound-guided hydrodissection of the posterior femoral cutaneous nerve at obturator canal at each visit, alternating right and left sides throughout the protocol (Figure 4). For the first treatment on each side, 2mL of dexamethasone with 5 mL of 1% lidocaine was placed around each nerve. At the following visits, 2 mL of Traumeel® [13,14] with 5 mL of 1% Lidocaine was used for the peripheral nerve hydrodissection. Patients continued to attend pelvic floor physical therapy at a facility of their choice throughout the protocol.
The pelvic floor physical therapy included the internal and external myofascial release of the pelvic floor musculature, scar tissue mobilization, visceral mobilization, skin rolling along the lower abdomen and buttocks, nerve gliding along the pudendal nerve and the posterior femoral cutaneous nerve, and diaphragmatic breathing.
A paired t-test assuming equal variances was used (SPSS®, Version 26) to determine statistical significance (α=0.05) of score changes. Descriptive Statistics was used to determine the lower and higher values of the Confidence Interval.
Outcome Measures
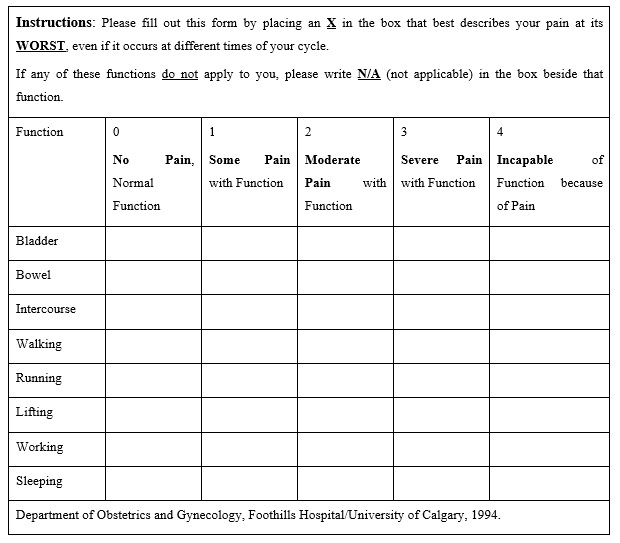
Response to treatment was measured before treatment and 6 weeks after treatment, using the 0 to 10 Visual Analogue Scale (VAS) to quantify pelvic pain and the Functional Pelvic Pain Scale (FPPS) to assess function. For the VAS, the patients were asked to rate their average pain intensity over the past 24 hours. Patients score pelvic function on the FPPS for eight categories: bladder, bowel, intercourse, walking, sleeping, working, running, and lifting (Figure 5). The patients rated each category from 0 to 4, with 0 for normal function, and 4 for severe debilitation. Thus, each patient was given a total pelvic function score between 0 and 32.
Results
73 female patients underwent ultrasound-guided, pelvic floor trigger-point injections, and peripheral nerve hydrodissection. Patients were able to return to work the same day as the procedure. No adverse events were noted. Follow-up data was measured up until 14 weeks post-treatment. The mean age of the participants was 35.5 years (SD 11.3) and the mean duration of pelvic pain was 5.8 years (SD 5.3). The results are shown in Table 2, noting statistically significant improvement in both intercourses and working. The rest of the subcategories were not statistically significant. Pretreatment, the mean VAS score was 6.8 [SD] 2.38); P < .05, 95% confidence interval (CI) 6.25 to 7.35. Post treatment, the mean VAS score was 5.08, (SD 2.67); P < .05, 95% confidence interval (CI) 4.46 to 5.70. The mean total FPPS score before treatment was 11.53 (Standard deviation [SD] 6.50); P < .05, 95% confidence interval (CI) 10.02 to 13.03. Post treatment, the mean FPPS score was 8.69, (SD 6.38); P < .05, 95% confidence interval (CI) 7.21 to 10.17. Analysis of the subcategories within the FPPS indicated that the improvement was statistically significant in the categories of intercourse and working. In the category of intercourse, the mean change in score after treatment was .72. Pretreatment, the mean was 2.01 (P < .05, 95% CI1.63-2.40). Post treatment, the mean was 1.29 (P < .05, 95% CI.96-1.63). In the category of work, the mean change in score after treatment was .62. Pretreatment, the mean was 2.08 (P < .05, 95% CI1.73-2.42). Post treatment, the mean was 1.46 (P < .05, 95% CI1.18-1.74).
Discussion
Our study evaluated the effectiveness of ultrasound-guided, pelvic floor trigger point injections and peripheral nerve hydrodissection, in conjunction with physical therapy, in female patients with a history of CPPS. Both the mean VAS and FPPS scores decreased significantly by 1.72 points and 2.84 points, respectively as shown in Figure 6. In this study, several aspects of pelvic pain and dysfunction improved after treatment with our protocol. Statistically significant improvements occurred for the categories of intercourse and working as shown in Figure 7. The treatment was safe, and we observed no immediate complications.
The protocol was developed for patients with CPPS to address the etiological triad of myofascial pain, peripheral neurogenic inflammation, and central sensitization [15,16]. The approach is threefold. First, we aim to decrease the spontaneous ectopic activity of peripheral nociceptors in the pudendal and posterior femoral cutaneous nerves with repetitive exposure to lidocaine 1% [17]. With this process, we are desensitizing the Nav1.7 channels involved in the aberrant firing of peripheral nociceptors [18]. Second, we aim to increase blood flow to the peripheral nerves by lyses of connective tissue restrictions with hydro-dissection [19]. Neurogenic inflammation is treated both with reversing the neural ischemia and using the medication dexamethasone one time on each side [20]. Third, we aim to reset short, spastic, and weak muscle spindles with trigger point injections to each muscle in the levator ani sling [21,22].
Most of the benefit of the injection protocol is mechanical in nature. However, the repetitive exposure to the anesthetic Lidocaine 1% is also crucial to reset hyperactive peripheral nociceptors and decrease the mast cell release of histamine [23]. Conceptually, creating space and increasing blood flow via lysis of fascial restrictions and release of constricting hypertonic muscular spasms [24] creates a better environment for the pelvic nerves to then heal themselves. Decreasing myofascial spasm and neurogenic inflammation, will ultimately reverse peripheral sensitization and subsequently decrease central sensitization as central pain processing is maintained by afferent nociceptive input [25].
One potential mechanism contributing to why patients are responding to the injection protocol is that we are creating space along the course of both the pudendal and posterior femoral cutaneous nerves by hydro-dissecting both Alcock's and Obturator canal using the supine and prone approach. There is a significant overlap in terms of pain patterns and innervation with the pudendal and posterior femoral cutaneous nerve [26]. Given their proximity and the cross-sensitization that occurs in the pelvis, the pudendal nerve, and the posterior femoral cutaneous nerve upregulate one another. Cross-sensitization involves noxious stimuli from an affected pelvic structure being transmitted to an adjacent, normal structure, causing functional changes such as sensitization in the latter [27]. Therefore, it is important to treat both the pudendal and posterior femoral cutaneous nerves simultaneously.
Absenteeism is a large burden on this patient population, and our study demonstrates CPPS patients' ability to return to work after treatment. One study analyzed 1,418 premenopausal women, without a previous surgical diagnosis of endometriosis, having a laparoscopy to investigate symptoms. Each affected woman lost on average 10.8 hours of work weekly, mainly owing to reduced effectiveness while working [28]. Another study that analyzed 810 women with endometriosis demonstrated significant decreased productivity and absenteeism noted at both work and at home [29].
27.9% of our patients had a pathological diagnosis of endometriosis. Deep dyspareunia occurs in 50% of women with endometriosis [30]. One study on women with endometriosis demonstrated that the severity of deep dyspareunia was strongly associated with pelvic floor tenderness and painful bladder syndrome, independent of endometriosis-specific factors. This study suggested that myofascial pain and nervous system sensitization plays an important role in deep dyspareunia in women with endometriosis [31]. Our study supports this theory, given the statistically significant improvement in the ability of patients to return to intercourse after treating their myofascial and nervous system dysfunction with our protocol.
Some limitations of our study include a short follow up time and a lack of a control group. In addition, the retrospective nature of this study is limiting but sets the stage for a prospective trial in the future.
Conclusion
This study demonstrated statistically significant positive outcomes for women with Chronic Pelvic Pain Syndrome who were treated with external ultrasound-guided trigger point injections to the pelvic floor musculature in combination with peripheral nerve hydrodissection and pelvic floor physical therapy. The alleviation of pain and improvement in function allowing patients to return to work and resume intercourse after treatment was particularly promising.
- (2020) Classification of chronic pain, second edition (Revised). IASP
- Stanford E, Koziol J, Fend A (2005) The prevalence of interstitial cystitis, endometriosis, adhesions, and vulvar pain in women with chronic pelvic pain. J Minim Invasive Gynecol 12: 43-49.
- Carey ET, Till SR, As-Sanie S (2017) Pharmacological management of chronic pelvic pain in women. Drugs. 77: 285–301.
- Speer L, Mushkbar S, Erbele T (2016) Chronic pelvic pain in women. Am Fam Physician 93: 380-387.
- Ortiz D (2008) Chronic pelvic pain in women. Am Fam Physician 77: 1535-1542.
- Whitaker LH, Reid J, Choa A (2016) An exploratory study into objective and reported characteristics of neuropathic pain in women with chronic pelvic pain. PLoS One 11: e0151950.
- Pastore EA, Katzman WB (2012) Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J Obstet Gynecol Neonatal Nurs 41: 680‐691.
- Dos Bispo AP, Ploger C, Loureiro AF, et al. (2016) Assessment of pelvic floor muscles in women with deep endometriosis. Arch Gynecol Obstet 294: 519-523.
- Montenegro ML, Vasconcelos EC, Candido Dos Reis FJ, Nogueira AA, Poli-Neto OB (2008) Physical therapy in the management of women with chronic pelvic pain. Int J Clin Pract 62: 263-269.
- Donnez J, Chantraine F, Nisolle M (2002) The efficacy of medical and surgical treatment of endometriosis-associated infertility: arguments in favor of a medico-surgical approach. Hum Reprod Update 8: 89–94.
- J Abbott, J Hawe, D Hunter, M Holmes, P Finn, R Garry (2004) Laparoscopic excision of endometriosis: A randomized, placebo-controlled trial. Fertility and Sterility 82: 878-884.
- Plavnik K, Tenaglia A, Hill C, Ahmed T, Shrikhande A (2019) A novel, non‐opioid treatment for chronic pelvic pain in women with previously treated endometriosis utilizing pelvic floor musculature trigger point injections and peripheral nerve hydrodissection.
- Porozov S, Cahalon L, Weiser M, Branski D, Lider O, Oberbaum M (2004) Inhibition of IL-1beta and TNF-alpha secretion from resting and activated human immunocytes by the homeopathic medication Traumeel S. Clin Dev Immunol 11: 143‐149.
- Schneider C (2011) Traumeel – an emerging option to nonsteroidal anti-inflammatory drugs in the management of acute musculoskeletal injuries. Int J Gen Med. 4: 225-234.
- Malykhina AP (2007) Neural mechanisms of pelvic organ cross-sensitization. Neuroscience 149: 660‐672.
- Bedaiwy MA, Patterson B, Mahajan S (2013) Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 58: 504-510.
- F Tu, K Hellman, M Backonja (2011) Gynecologic management of neuropathic pain. American Journal of Obstetrics and Gynecology 435-443.
- Ivy E Dick 1, Richard M Brochu, Yamini Purohit, Gregory J Kaczorowski, et al. (2007) Sodium Channel Blockade May Contribute to the Analgesic Efficacy of Antidepressants. J Pain. 8: 315-24.
- Andrea Trescot (2015) Michael Brown. Peripheral Nerve Entrapment, Hydrodissection, and neural regenerative strategies. Techniques in regional anesthesia and pain management 19: 85- 93.
- Megumi Matsuda, Yul Huh, Ru-Rong Ji (2019) Roles of Inflammation, Neurogenic inflammation, and Neuroinflammation in Pain. J Anesth. 33: 131–139.
- Nicholas N. Tadros, Anup B. Shah, and Daniel A. Shoskes (2017) The utility of trigger point injection as an adjunct to physical therapy in men with chronic prostatitis/chronic pelvic pain syndrome. Transl Androl Urol. 6: 534–537.
- Bartley J, Han E, Gupta P, Gaines N, Killinger KA, et al. (2018) Transvaginal trigger point injections improve pain scores in women with pelvic floor hypertonicity and pelvic pain conditions. Female Pelvic Med Reconstr Surg.
- H Yanagi, H Sankawa, H Saito , Y Ikura (1996) Effect of lidocaine on histamine release and Ca2+ mobilization from mast cells and basophils. Acta Anesthesiol Scand 40: 1138-1144.
- S Prendergrast, J Weiss (2003) Screening for Musculoskeletal Causes of Pelvic Pain. Clinical Obstetrics and Gynecology 46: 773-782.
- Staud R, Nagel S, Robinson ME, Price DD (2009) Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebocontrolled study. Pain 145: 96‐104.
- Natalia Murinova, Daniel Krashin, and Andrea M Trescot (2016) Posterior Femoral Cutaneous Nerve Entrapment: Low Back. Peripheral Nerve Entrapments: Clinical Diagnosis and Management, Springer International Publishing Switzerland.
- Aredo J, Heyrana K, Karp B, Shah J, Stratton P (2017) Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction. Semin Reprod Med. 35: 88-97.
- K. E. Nnoaham, L Hummelshoj, P Webster, T d’Hooghe, F de Cicco Nardone, C de Cicco Nardone,C Jenkinson, S H. Kennedy, K T. Zondervan (2011) Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries Fertil Steril. 96: 366–373.e8.
- AM Soliman, K Coyne , K S Gries , J Castelli-Haley , M C Snabes , ES Surrey (2017) The Effect of Endometriosis Symptoms on Absenteeism and Presenteeism in the Workplace and at Home. J Manag Care Spec Pharm. 23:745-754.
- L K. Shum, M A. Bedaiwy, MD, C Allaire, C Williams, H Noga, A Albert, S Lisonkova, MD, P J. Yong (2018) Deep Dyspareunia and Sexual Quality of Life in Women With Endometriosis. Sex Med. 6: 224–233.
- N L Orr, H Noga, C Williams, C Allaire, M A Bedaiwy, S Lisonkova, K B Smith, P J Yong (2018) Deep Dyspareunia in Endometriosis: Role of the Bladder and Pelvic Floor. J Sex Med 15 :1158-1166.

Tables at a glance