Comparing two Different Protocols of Ovulation Trigger in Polycystic Ovary Syndrome Patients Undergoing Intracytoplasmic Sperm Injection
Received Date: April 28, 2020 Accepted Date: May 14, 2020 Published Date: May 17, 2020
doi: 10.17303/jwhg.2020.7.204
Citation: Mervat Sheikh Elarab (2020) Comparing two Different Protocols of Ovulation Trigger in Polycystic Ovary Syndrome Patients Undergoing Intracytoplasmic Sperm Injection. J Womens Health Gyn 7: 1-13.
Abstract
Background: Polycystic ovary syndrome (PCOS) patients who are candidates for intracytoplasmic sperm injection (ICSI) are known to have a narrow threshold ceiling for gonadotropins than normal women making them vulnerable to rapidly developing ovarian syndrome (OHSS).
Objective: The study aims at evaluating the use of gonadotropin-releasing hormone (GnRH) agonist as a final oocyte maturation trigger in comparison to human chorionic gonadotropin (hCG) in PCOS patients undergoing ICSI using GnRH antagonist stimulation protocol.
Methods: It is a prospective randomized study, including 112 PCOS patients undergoing ICSI between September 2018 and July 2019 in El-Shatby Maternity University hospital and a private center. A GnRH antagonist stimulation protocol was used in all patients then final oocyte maturation triggering was achieved by either hCG (Group A) (n=56) or GnRH agonist (Group B) (n=56). Each group was then divided into two subgroups which either got all their embryos frozen (A1 and B1, n=29) or underwent fresh embryo transfer (A2 and B2, n=27).
Results: The rate of development of OHSS differed significantly between these two groups. It was higher when the hCG trigger was used (p< 0.001). Despite a statistically significant higher number of oocytes retrieved (p=0.019), more MII oocytes (p< 0.001), and higher fertilization rate in the hCG group (p< 0.001), no statistically significant difference was encountered when the two groups were compared regarding the implantation rate (p=0.322) or the clinical pregnancy rate (CPR) (p=0.788).
Conclusion: In PCOS patients undergoing ICSI using GnRH antagonist stimulation cycles, the GnRH agonist trigger is considered a better alternative to hCG in order to minimize the risk of development of OHSS as much as possible without compromising the pregnancy rate.
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous multifactorial disorder that is very common in women of the reproductive age. It is actually considered the commonest endocrine disorder of this age group [1]. The definition and diagnosis of PCOS are not straight forward; that is why certain criteria should be present before declaring or managing PCOS. The so-called "Rotterdam criteria", being the most widely used though not universally accepted, were created by the members of the American Society of Reproductive Medicine (ASRM) and the European Society of Human Reproduction and Embryology (ESHRE) in Rotterdam, Netherlands, in 2003. Three definitions for PCOS remain valid at present to aid the diagnosis and they include first the National Institute of Child Health and Human Development (NICHD) has an older definition (1990) that requires the presence of both; hyperandrogenism and ovulatory dysfunction without considering ovarian morphology, second, the Rotterdam criteria (2003) that propose that PCOS can be diagnosed in any woman presenting with at least two of the three following characteristics clinical (Ferriman-Gallwey Score ≥ 8) and/or biochemical hyperandrogenism (elevated total/free testosterone), ovulatory dysfunction and polycystic ovarian morphology (PCOM) by ultrasonography (diagnosed by finding ovarian volume ≥ 10 cm3 or counting ≥ 12 antral follicles in one ovary) and third, the Androgen Excess and PCOS Society (AE–PCOS) Position Statement (2006) that necessitated the presence of hyperandrogenism, which must be accompanied by evidence of ovarian dysfunction in the form of ovulatory dysfunction and/or PCOM [1-3].
The prevalence of PCOS differs according to the studied population and which diagnostic criteria are used and it ranges between 5 and 20% [4]. Being the main cause of anovulatory infertility, PCOS management is primarily dependent upon ovulation induction [5-7]. The main risk of controlled ovarian stimulation (COS) in PCOS patients is the development of ovarian hyperstimulation syndrome (OHSS) which is an iatrogenic complication that is known to be life-threatening when severe [8, 9]. PCOS patients characterized by having higher anti-mullerian hormone (AMH) levels are more liable to develop OHSS during controlled ovarian stimulation (COS) [10, 11]. Another potential risk is the development of multiple pregnancies; especially high order pregnancy [8].
When simple ovulation induction methods fail e.g. letrozole or clomiphene citrate, or when other infertility factors exist e.g. tubal factor or male factor infertility; then it is time for the introduction of assisted reproductive techniques (ART); which entails in vitro fertilization (IVF) or intracytoplasmic sperm injection [5-7, 12].
PCOS patients are known to require higher FSH doses but they are also known for their low threshold ceiling thus rapidly progressing to OHSS. Therefore, choosing the stimulation protocol, adjustment of the dose of gonadotropins and the choice of the final oocyte maturation trigger is challenging and critical [8, 13].
The GnRH antagonist protocol is now the most common and the best stimulation protocol used in PCOS patients as it lowers the risk of OHSS without impairing the clinical pregnancy rate (CPR), ongoing pregnancy rate (OPR) or the live birth rate (LBR) [11, 14-17]. This is strongly recommended by the American College of Obstetricians and Gynecologists (ACOG) as grade A evidence [15].
Individualization of the dose of the used gonadotropins and their early administration allows for more coherent follicular recruitment; this reduces the variability in follicular size on the day of administration of the oocyte maturation trigger and increases the number of retrieved mature oocytes [18].
Advantages of the antagonist protocol include achieving more immediate gonadotropin suppression, requiring fewer doses of exogenous gonadotropins, resulting in a shorter stimulation period and causing less incidence of OHSS [11, 14, 16, 17, 19]. Final oocyte maturation is a crucial step to retrieve mature oocyte ready to subsequent laboratory processing [20] Choosing the GnRH antagonist stimulation protocol also allows achieving final oocyte maturation using either human chorionic gonadotropin (hCG) or GnRH agonist [20].
The GnRH agonist trigger can displace the antagonist at the GnRH receptors thus providing a controlled LH surge. The endogenous GnRH agonist-induced LH has a shorter half-life than hCG causing less autotrophic stimulation thus reducing the risk of OHSS [11, 14, 16, 17, 19].
Due to early luteolysis, it was previously thought that the patient will encounter a severe luteal phase defect (LPD) within 5 days of using the agonist trigger, but recently it was found that the degree of luteolysis and LPD is patient-specific. Yet unfortunately, there are no available parameters to estimate the degree of luteolysis. [17, 20-22].
Owing to the shorter half-life of GnRH agonist trigger causing early luteolysis; it has been claimed than using it as a trigger instead of hCG would reduce the pregnancy rates and the live birth rates so different ways to support the luteal phase had emerged to overcome these unfavorable results e.g. dual triggering, luteal coasting and/or adding hCG or estrogen to the standard doses of progesterone for luteal phase support (LPS) or freezing all embryos for transfer in a subsequent cycle [23-25].
Therefore, this study was conducted aiming at evaluating the use of GnRH agonist as a final trigger of oocyte maturation in comparison to hCG in PCOS patients undergoing ICSI using GnRH antagonist stimulation protocol to lower the risk of OHSS. The aim of the study was achieved.
Methods
Setting: El-Shatby Maternity University hospital and a private center.
Study design: This was a prospective randomized study conducted between September 2018 and July 2019.
All included women underwent a fixed GnRH antagonist protocol of COS then on the day of the trigger, they were randomly distributed into two study groups using computer-based randomization (Random Digit Software). The institutional review board (IRB) approval was obtained. Before starting ovulation induction, all patients were the required evaluation was performed to confirm the presence of inclusion criteria and the absence of the exclusion criteria.
On the second day of the menstrual cycle (whether spontaneous or induced), ovulation induction was initiated only when serum estradiol (E2) level obtained on that day was less than 50 pg/dl and no follicular activity was seen on TVUS. Induction was initiated by the administration of recombinant follicle-stimulating hormone (recFSH) (gonal-F) and human menopausal gonadotropin (HMG) (Menogon) in a total dose of 150-225 IU daily for 5 days then the patient came for her first follow up visit to assess the degree of elevation of serum E2, the thickness and pattern of the endometrium and the size and number of the growing follicles. GnRH antagonist; cetrorelix acetate 0.25 mg (cetrotide) was given daily starting on stimulation day 6- regardless of the size of the dominant follicle- by subcutaneous route “fixed antagonist protocol” to suppress endogenous luteinizing hormone [27, 28].
Follow up was done repeatedly every two days with ultrasonic and E2 analysis and the doses of recFSH and HMG were adjusted according to the individual response of each patient with possible dose increments of 75 IU till three leading follicles reach 17 mm or more in size, then serum progesterone level was tested and the trigger was given [27, 29]. Oocyte retrieval was performed by ultrasound-guided vaginal follicle aspiration under a strictly aseptic technique 36 hours after giving the trigger [27, 29].
Two main groups were created depending on the trigger protocol used:
Group A: 56 subjects were triggered by 5000 IU of hCG given intramuscularly.
• Group A1: In 27 subjects, all embryos were frozen and no further treatment was given.
• Group A2: 29 subjects underwent a fresh embryo transfer. Luteal phase support was performed by using progesterone suppositories 400 mg given twice daily (Prontogest) and progesterone intravaginal gel 90 mg given once daily (Crinone) starting on the day of oocyte retrieval.
Group B: 56 subjects were triggered by GnRH agonist (0.2 mg of decapeptyl) administered subcutaneously.
• Group B1: In 27 subjects, all embryos were frozen, and also no further treatment was given.
• Group B2: 29 subjects underwent fresh embryo transfer with intense luteal phase support adding estrogen 4 mg orally (two white tablets of cycloprogenova) to the same progesterone regimen as group A2.
All patients were closely followed and cases who developed OHSS were closely monitored and managed according to the severity of the condition. Oocyte quality was assessed in the ICSI laboratory, oocytes of good quality were injected with sperms then fertilized oocytes were followed till the day of embryo transfer and/or embryo freezing and the quality of embryos was also assessed and documented.
In the fresh embryo transfer groups, only 1-2 blastocysts were transferred in case of the day 5 transfer while in case of day 3 transfer (cleavage stage embryos), 2-4 embryos were transferred. Patients who underwent fresh embryo transfer were followed beyond the time of serum pregnancy testing and a serum β-hCG (beta subunit of hCG) level of >5 IU/mL was used for diagnosis of a successful biochemical pregnancy. Clinical pregnancy was confirmed when a gestation sac with evident fetal heartbeats was detected upon ultrasonography. On the other hand, patients who had all their embryos cryopreserved received no luteal phase support and were followed till menses issued. The main outcome measures in the fresh embryo transfer cycles of this study were the implantation rate, the clinical pregnancy rate (CPR) and the occurrence and severity of OHSS while the secondary outcome measures entailed the number of the retrieved oocytes, their quality and the number and quality of fertilized embryos.
Results
Data were fed into the computer and they were subsequently analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) [30]. Qualitative data were described in terms of number and percentage. The Kolmogorov-Smirnov test was the test used to verify the normality of distribution. Quantitative data were described in terms of mean, median, range (minimum and maximum) and standard deviation [1]. The significance of the obtained results was judged at the 5% level.
Descriptive Analysis
Comparison according to Demographic Data
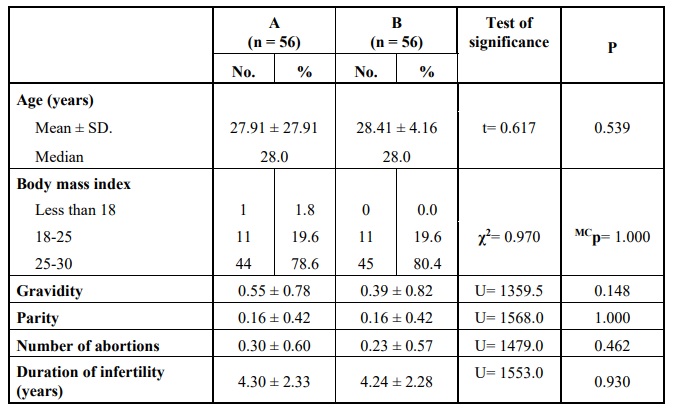
Comparing the two groups according to the demographic data, showed no statistically significant difference Table 1.
Comparison according to Laboratory Investigations
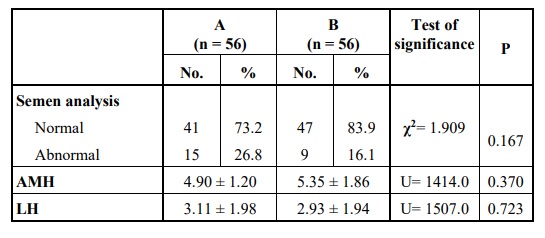
Also, no statistically significant difference was detected on comparing the two groups regarding their partners’ semen analysis, their AMH level, or LH level Table 2.
Comparative Analysis
Comparison according to the Details of Stimulation
There was no statistically significant difference obtained when the two groups were compared according to the number of stimulation days or the daily gonadotropin dose needed for stimulation Table 3.
The used tests were:
• Chi-square test for categorical variables, to compare between different groups. • Fisher’s Exact or Monte Carlo correction for chi-square when more than 20% of the cells have expected count less than 5. • Student t-test for normally distributed quantitative variables, to compare between two studied groups. • Mann Whitney test for abnormally distributed quantitative variables, to compare between the two studied groups.
Comparison according to Ultrasound Findings
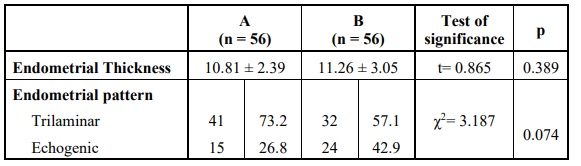
No statistically significant difference was found on comparing the two groups regarding the endometrial thickness or the endometrial pattern detected on the day of oocyte retrieval Table 5.
Outcome Statistics
Comparison according to ICSI Laboratory Findings
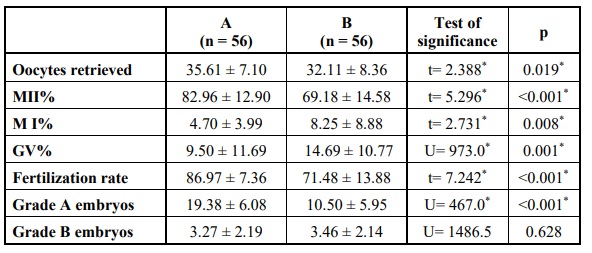
More oocytes were retrieved in group A with more mature oocytes in group A and more MI and GV oocytes in group B and the differences were statistically significant. Also, the fertilization rate and good quality embryos were significantly higher in group A Table 6.
Comparison according to OHSS
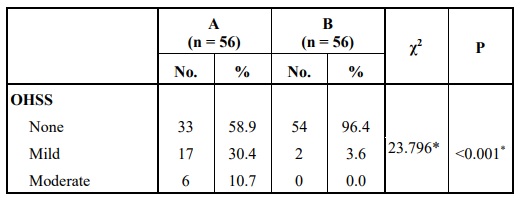
Comparing the two groups with respect to the risk of development of OHSS, showed a statistically significantly higher OHSS development rate when hCG was used for oocyte maturation triggers Table 7.
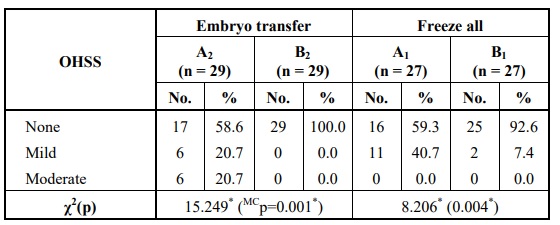
In the case of fresh embryo transfer, the number of patients who developed OHSS after receiving hCG trigger was higher than those who received the GnRH agonist trigger and the difference was statistically significant. The same was encountered when the two "freeze-all" subgroups. Fortunately, all cases who developed OHSS had either a mild or a moderate form so they were managed at home without requiring hospitalization Table 8.
Comparison between the two Embryo Transfer Subgroups
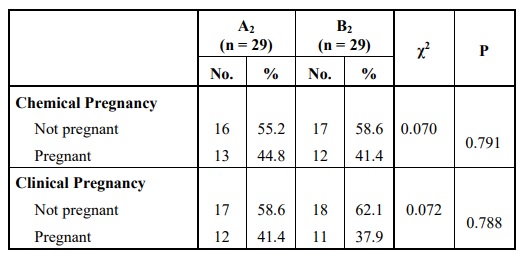
No statistically significant difference was found in comparing both groups according to chemical or clinical pregnancies although the clinical pregnancy rate was higher in group A2 Table 9. Comparing the two groups regarding the implantation rate revealed no statistically significant difference Table 10.
Comparison between the two Freeze-All Subgroups
In group A1, patients got their menses within 7-14 days after oocyte retrieval while in group B1, they got their menses within 7-16 days. Comparing the two groups according to the timing of menses revealed no statistically significant difference Table 11.
Discussion
This randomized prospective study was conducted aiming at finding out whether using hCG or GnRH agonists for oocyte maturation could affect the outcome in PCOS patients undergoing ICSI using the antagonist stimulation protocol. The study enrolled 112 PCOS patients. They were randomly allocated to receive either hCG or GnRH agonist as an oocyte maturation trigger. Each group was then divided into two subgroups; either freshly transferring embryos with or without freezing surplus embryos or freezing all embryos for transfer in a subsequent cycle.
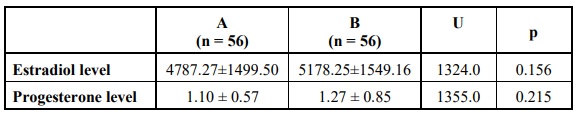
Comparing the two main groups revealed no statistically significant difference regarding the demographic data (including age, body mass index, gravidity, parity or the number of abortions), the duration and causes of infertility or laboratory investigations (semen analysis of the patients’ partners, AMH level, LH level, the maximum level of estradiol reached at the end of stimulation and the level of serum progesterone measured on the day the trigger). Also, the details of stimulation including the dose and duration of stimulation were not significantly different.
In the freeze-all subgroups; there was no statistically significant difference when the two groups where compared in relation to the timing of menses. This denotes that using the GnRH agonist trigger does not cause significant luteolysis or predispose to severe luteal phase defect. On the other hand, the results revealed a statistical difference when using hCG for triggering oocyte maturation that led to a significantly higher number of retrieved oocytes (p = 0.019), more mature oocytes collected, more MII (p < 0.001) and less MI (p = 0.008) and GV oocytes (p = 0.001) and a higher fertilization rate (p < 0.001) with more grade A embryos (p< 0.001) produced but no difference in grade B produced embryos. This significant difference in oocyte yield is considered of little clinical significance since PCOS patients have no problems with the number of oocytes.
However, more patients in this group developed OHSS (p< 0.001). Since we used 5000 IU of hCG instead of 10,000 IU, it was also concluded that even using a smaller dose of hCG cannot eliminate the risk of OHSS but can only reduce the severity as there were no cases that developed severe forms of OHSS. Using 5000 IU of hCG was also enough to achieve final oocyte maturation and retrieve a large number of oocytes (significantly higher than GnRH agonist), so this can be considered to reduce the risk of OHSS developing in normal and high responder patients.
Yet, there were no detected statistically significant differences between the two groups regarding the implantation rate (p = 0.322) or the CPR (p = 0.788). So, it was concluded that using the GnRH agonist trigger in PCOS patients undergoing antagonist protocol is a very good alternative that reduces the risk of OHSS without compromising the pregnancy outcomes.
Being a major concern due to the higher vulnerability to develop OHSS, PCOS has been the target of many studies in the literature. Most of these studies aimed at minimizing the risk of OHSS. Engmann et al (2006) performed a retrospective study on high responders mainly PCOS patients undergoing IVF/ICSI. Analysis of the results showed that no statistically significant difference was elicited between the two groups when compared in respect to the dose of gonadotropins required for stimulation, the mean number of retrieved oocytes, the proportion of mature oocytes, their fertilization rate or the number and quality of the transferred or frozen embryos. Comparing both groups regarding the rate of occurrence of OHSS, the implantation rate, the clinical pregnancy rate, and the birth rate also revealed no statistically significant difference. They concluded that GnRH agonist is an effective trigger of oocyte maturation in PCOS patients and high responders [26]. The results of this study matched our results in considering GnRH agonists trigger an option for high responders as it does not reduce the implantation rate or the CPR but in contradiction to our study, there was no difference in the occurrence of OHSS. Their study is criticized for being a retrospective study, including a small number of patients (23 in each arm), using different stimulation protocols (agonist versus antagonist) and even the dose of hCG trigger (3300-10,000 IU) was not standardized.
Kovachev E, et al. (2008) conducted a study aiming at evaluating the use of GnRH agonist trigger for achieving final oocyte maturation in PCOS patients undergoing ART. GnRH antagonist stimulation protocol was used. Only one patient developed severe, late OHSS. So, they concluded that using GnRH agonist as a maturation trigger is a good alternative in PCOS patients as it helps to reduce the risk of OHSS without affecting the CPR (which was 46%) [31]. Again the results of this study were in agreement with our results. Also, the only case of late OHS might indicate that the freeze-all policy is a better alternative. However, the study is criticized for its small sample size (29 patients) and the lack of a control group.
Krishna D, et al. (2016) performed a prospective, randomized controlled trial on 227 PCOS patients undergoing ICSI using the GnRH antagonist stimulation protocol. No cases in the
study group developed moderate to severe OHSS while 37.6% of the control group developed moderate to severe OHSS which was statistically significant. Comparing the two groups also revealed a significantly larger number of retrieved oocytes (19.1 ± 11.7 vs. 14.1 ± 4.3), a higher amount of fertilized oocytes (15.6 ± 5.6 vs. 11.7 ± 3.6) and better quality of day 3 cleavage- stage embryos (12.9 ± 4.7 vs. 7.5 ± 4.3) in the agonist rather than the hCG group. They concluded that using GnRH agonist trigger in these patients rather than hCG significantly lowers the risk of OHSS, yields more mature oocytes, higher fertilization rates and produces embryos of better quality and is therefore recommended [32]. This study has the advantages of including large sample size, being prospective and randomized. It was almost similar to our study and reached the same conclusion but they differed in using recombinant hCG, in the incidence of moderate-to-severe OHSS which was remarkably high and in finding out that using GnRH agonist trigger revealed more oocytes, more fertilized embryos, and better-quality embryos than hCG trigger. This finding contradicts most of the literature in the report. Furthermore, despite that 250 μg of recombinant hCG are considered equivalent to 5000 IU of hCG, this difference cannot be entirely disregarded. Haahr T et al (2017) conducted a systematic review and meta-analysis including studies published till December 2016 (859 infertile couples) using the GnRH antagonist stimulation protocol, comparing administration of hCG trigger versus GnRH agonist trigger and having fresh embryo transfer. There was no statistically significant difference between both groups when they were compared for the live birth rate (LBR) (OR 0.84, 95% CI 0.62, 1.14) or OHSS (4/413 in the agonist group and 7/413 in the hCG group) (OR 0.48, 95% CI 0.15, 1.60). Comparing them according to the miscarriage rate revealed a higher but non-statistically significant rate in the GnRH agonist group (OR 1.85; 95% CI 0.97, 3.54). Therefore they concluded that GnRH trigger is comparable to hCG trigger as long as luteal phase support is individualized [25].
Yilmaz N et al (2019) conducted a study aiming at evaluating GnRH agonist trigger in 36 high responder patients undergoing IVF/ICSI who used the GnRH antagonist stimulation protocol versus a historical control (n=15). All cycles had a fresh embryo transfer. Comparing both groups in respect to the number of mature oocytes (MII) or the fertilization rate was not statistically significant, neither was the rate of blastocyst formation or the clinical pregnancy rate. They concluded that using GnRH agonist to trigger final oocyte maturation is a better alternative to hCG thus virtually eliminating the risk of OHSS; without compromising the pregnancy outcomes [33]. This study again agrees with our study although the sample size was small and the historical control group represented less than half of the sample size.
Jones BP et al (2019) conducted a study on oocyte donors undergoing controlled ovarian stimulation using GnRH antagonist protocol using either hCG, agonist or dual trigger. Comparing the three groups according to the number of mature oocytes revealed a significantly lower number in the hCG group than the GnRH agonist and the dual trigger groups. The OHSS rate was significantly higher in the dual trigger group (5 patients i.e. 8.5%) than the GnRH agonist group (one case i.e. 0.4%) and the hCG group (0%). So, they concluded that the GnRH agonist trigger significantly reduces the risk of OHSS in normal and high responders while maximizing the yield of mature oocytes. They also recommended using hCG triggers in low responders who are at low risk for OHSS as the GnRH agonist trigger can cause lower pregnancy rates [34]. In this study, the hCG trigger was used only in low responders, that is why no cases of OHSS developed in this arm and when the dual trigger was used in normal and high responders, more OHSS developed in the dual trigger group. In addition, using different populations increased the confounding factors and the study was criticized for not providing any data about embryo quality, blastulation, or pregnancy rate.
In contradiction to our study, Youssef MAFM et al (2011) conducted a meta-analysis including 11 RCTs (involving 1055 women) aiming at comparing the use of GnRH agonist versus hCG for oocyte maturation triggering in infertile patients undergoing IVF/ICSI using the GnRH antagonist protocol. They found that using GnRH agonist trigger resulted in lower live birth rates (OR 0.44, 95% CI 0.29 to 0.68; 4 RCTs), lower ongoing pregnancy rates (OR 0.45, 95% CI 0.31 to 0.65; 8 RCTs), lower OHSS rate (OR 0.10, 95% CI 0.01 to 0.82; 5 RCTs) and higher miscarriage rates when compared to hCG trigger in autologous cycles so they recommended against using GnRH agonist trigger routinely in GnRH antagonist stimulation cycles and that it should only be used in patients at high risk of developing OHSS. In donor cycles, no statistically significant differences had been obtained between GnRH agonist and hCG triggers. (35) The ongoing pregnancy rates and lower live birth rates may point to the importance of individualization of luteal phase support especially when using the GnRH agonist trigger (luteal coasting) [17, 20-22].
Although meta-analysis is considered on the top of the pyramid of evidence, this meta-analysis is relatively old (2011); including studies conducted behind the time when the learning curve of the agonist trigger was still rising. So, despite the high statistical power of this meta-analysis because of pooled data of the included studies, their conclusions are negatively affected by the heterogeneity of the studied populations.
In conclusion, there is a strong agreement that using the GnRH agonist trigger to achieve final oocyte maturation is a better alternative than hCG trigger in PCOS patients undergoing ICSI to reduce the risk of OHSS without altering the pregnancy rates.
Our study has the advantage of being a prospective randomized study, that included a reasonably large sample size than that calculated statistically (50). The results showed that it is better to use GnRH agonist to trigger final oocyte maturation rather than hCG in order to minimize the risk of OHSS while at the same time maintaining the CPR, even though we used a smaller dose of hCG (5000 IU instead of 10,000 IU). It also revealed that GnRH agonist trigger is not a major cause of luteal phase defect as was evidenced by finding no significant difference between the two main groups when they were compared for the timing of menses in freeze-all cycles who received no luteal phase support; this confirms the concept of individualization of luteal phase support by measuring the level of serum progesterone during the luteal phase and administering hCG only when progesterone level drops significantly (luteal coasting).
However, the study ended when a clinical pregnancy was diagnosed and did not follow these patients to assess the rate of late OHSS, the miscarriage pregnancy rates, or live birth rates. It did not also follow the freeze-all cycle patients to assess whether embryo freezing can affect the results so we recommend the development of other studies with longer follow up to assess these parameters.
Conclusion
It was concluded that using GnRH agonist to trigger final oocyte maturation in PCOS patients undergoing ICSI using GnRH antagonist stimulation cycles is a better alternative than hCG to minimize the risk of OHSS in these high responders without compromising the pregnancy rate. Another conclusion is that the GnRH agonist trigger does not cause major LPD and therefore LPS should be individualized.
Acknowledgments
No financial support was gained from any organization and no one contributed to this work other than the authors.
- Escobar-Morreale HF (2018) Polycystic ovary syndrome: definition, aetiology, diagnosis, and treatment. Nature Reviews Endocrinology 14: 270.
- Wang R, Mol BWJ (2017) The Rotterdam criteria for polycystic ovary syndrome: evidence-basedd criteria? Human Reproduction 32: 261-264.
- Roe AH, Dokras A (2011) The diagnosis of polycystic ovary syndrome in adolescents. Reviews in obstetrics and gynecology 4: 45.
- Deswal R, Nanda S, Dang AS (2019) Single nucleotide polymorphisms in treatment of polycystic ovary syndrome: a systematic review. Drug metabolism reviews 1-11.
- Costello M, Garad R, Hart R, Homer H, Johnson L, Jordan C, et al. (2019) A Review of First Line Infertility Treatments and Supporting Evidence in Women with Polycystic Ovary Syndrome. Medical Sciences 7: 95.
- Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, et al. (2019) A brief update on the evidence supporting the treatment of infertility in polycystic ovary syndrome. Australian and New Zealand Journal of Obstetrics and Gynaecology.
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human Reproduction 33: 1602-1618.
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. (2016) The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Human reproduction update 22: 687-708.
- Nastri C, Teixeira D, Moroni R, Leitão V, Martins W (2015) Ovarian hyperstimulation syndrome: pathophysiology, staging, prediction, and prevention. Ultrasound in Obstetrics & Gynecology 45: 377-393.
- Tshzmachyan R, Hambartsoumian E (2019) The role of Letrozole (LE) in controlled ovarian stimulation (COS) in patients at high risk to develop ovarian hyperstimulation syndrome (OHSS). A prospective randomized controlled pilot study. Journal of Gynecology Obstetrics and Human Reproduction 101643.
- Eftekhar M, Bagheri RB, Neghab N, Hosseinisadat R (2018) Evaluation of pretreatment with Cetrotide in an antagonist protocol for patients with PCOS undergoing IVF/ICSI cycles: a randomized clinical trial. JBRA assisted reproduction 22: 238.
- Hafezi SG, Zand MA, Molaei M, Eftekhar M (2019) Dynamic model with factors of the polycystic ovarian syndrome in infertile women. International Journal of Reproductive Biomedicine 17.
- Sheng Y, Lu G, Liu J, Liang X, Ma Y, Zhang X, et al. (2017) Effect of body mass index on the outcomes of controlled ovarian hyperstimulation in Chinese women with polycystic ovary syndrome: a multicenter, prospective, observational study. Journal of assisted reproduction and genetics 34: 61-70.
- Lambalk C, Banga F, Huirne J, Toftager M, Pinborg A, Homburg R, et al. (2017) GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Human reproduction update 23: 560-579.
- Lin H-Y, Li Y, Wang W-J, Qiu Q, Zhang Q-X, Li Y (2019) Role of the proportion of dominant follicles in patients with polycystic ovary syndrome undergoing in vitro fertilization-embryo transfer. Chinese medical journal 132: 1448-1453.
- Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, et al. (2016) Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Human reproduction 31: 1253-1264.
- Lawrenz B, Samir S, Garrido N, Melado L, Engelmann N, Fatemi HM (2018) Luteal Coasting and Individualization of Human Chorionic Gonadotropin Dose after Gonadotropin-Releasing Hormone Agonist Triggering for Final Oocyte Maturation—A Retrospective Proof-of-Concept Study. Frontiers in Endocrinology 9:33.
- Abbara A, Patel AH, Hunjan T, Clarke S, Chia G, Eng PC, et al. (2019) FSH requirements for follicle growth during controlled ovarian stimulation. Frontiers in endocrinology 10: 579.
- Corbett S, Shmorgun D, Claman P, Cheung A, Sierra S, Carranza-Mamane B, et al. (2014) The prevention of ovarian hyperstimulation syndrome. Journal of Obstetrics and Gynaecology Canada 36: 1024-1033.
- Lawrenz B, Humaidan P, Kol S, Fatemi HM (2018) GnRHa trigger and luteal coasting: a new approach for the ovarian hyperstimulation syndrome high-risk patient? Reproductive biomedicine online 36: 75-77.
- Lawrenz B, Garrido N, Samir S, Ruiz F, Melado L, Fatemi HM (2017) Individual luteolysis pattern after GnRH-agonist trigger for final oocyte maturation. PloS one 12: e0176600.
- Kol S, Breyzman T, Segal L, Humaidan P (2015) 'Luteal coasting' after GnRH agonist trigger– individualized, HCG-based, progesterone-free luteal support in 'high responders': a case series. Reproductive biomedicine online 31: 747-751.
- Ding N, Liu X, Jian Q, Liang Z, Wang F (2017) Dual trigger of final oocyte maturation with a combination of GnRH agonist and hCG versus a hCG alone trigger in GnRH antagonist cycle for in vitro fertilization: a systematic review and meta-analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 218: 92-98.
- Engmann L, Maslow B, Kaye L, Griffin D, DiLuigi A, Schmidt D, et al. (2019) Low dose human chorionic gonadotropin administration at the time of gonadotropin releasing-hormone agonist trigger versus 35 h later in women at high risk of developing ovarian hyperstimulation syndrome–a prospective randomized double-blind clinical trial. Journal of ovarian research 12: 8.
- Haahr T, Roque M, Esteves SC, Humaidan P (2017) GnRH agonist trigger and LH activity luteal phase support versus hCG trigger and conventional luteal phase support in fresh embryo transfer IVF/ICSI cycles—a systematic PRISMA review and meta-analysis. Frontiers in endocrinology 8:116.
- Engmann L, Siano L, Schmidt D, Nulsen J, Maier D, Benadiva C (2006) GnRH agonist to induce oocyte maturation during IVF in patients at high risk of OHSS. Reproductive biomedicine online 13: 639-644.
- Decleer W, Osmanagaoglu K, Seynhave B, Kolibianakis S, Tarlatzis B, Devroey P (2014) Comparison of hCG triggering versus hCG in combination with a GnRH agonist: a prospective randomized controlled trial. Facts, views & vision in ObGyn 6: 203.
- Depalo R, Jayakrishan K, Garruti G, Totaro I, Panzarino M, Giorgino F, et al. (2012) GnRH agonist versus GnRH antagonist in vitro fertilization and embryo transfer (IVF/ET). Reproductive biology and endocrinology 10: 26.
- Papanikolaou EG, Humaidan P, Polyzos N, Kalantaridou S, Kol S, Benadiva C, et al. (2011) New algorithm for OHSS prevention. Reproductive Biology and Endocrinology 9: 147.
- Kirkpatrick LA, Feeney BC (2013). A simple guide to IBM SPSS statistics for version 20.0. Student ed. Belmont, Calif.: Wadsworth, Cengage Learning.
- Kovachev E (2008) Protocol with GnRH-antagonist and ovulation trigger with GnRH-agonist in risk patients--a reliable method of prophylactic of OHSS. Akusherstvo i ginekologiia 47: 16-19.
- Krishna D, Dhoble S, Praneesh G, Rathore S, Upadhaya A, Rao K (2016) Gonadotropin-releasing hormone agonist trigger is a better alternative than human chorionic gonadotropin in PCOS undergoing IVF cycles for an OHSS Free Clinic: A Randomized control trial. Journal of human reproductive sciences 9: 164.
- Yılmaz N, Ceran MU, Ugurlu EN, Gülerman HC, Engin Ustun Y (2019) GnRH agonist versus HCG triggering in different IVF/ICSI cycles of the same patients: a retrospective study. Journal of Obstetrics and Gynaecology 1-6.
- Jones BP, Al-Chami A, Gonzalez X, Arshad F, Green J, Bracewell-Milnes T, et al. (2019) Is oocyte maturity influenced by ovulation trigger type in oocyte donation cycles? Human Fertility 1-7.
- Youssef MA, Van der Veen F, Al‐Inany HG, et al. (2011) Gonadotropin‐releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles. Cochrane Database of Systematic Reviews.



Tables at a glance