Differences in EMG Activity of the Pelvic Floor Muscles in Women with Com- plaints of the Overactive Bladder Syndrome and Healthy Women
Received Date:April 18, 2020Accepted Date: May 17, 2020Published Date: May 19, 2020
doi: 10.17303/jwhg.2020.7.203
Citation: Bennink D (2020) Differences in EMG Activity of the Pelvic Floor Muscles in Women with Complaints of the Overactive Bladder Syndrome and Healthy Women. J Womens Health Gyn 7: 1-9.
Abstract
Objective: To determine the differences in EMG activity of the individual pelvic floor muscles during contractions and relaxation in patients with OAB compared to healthy volunteers to gain more insight in the pelvic floor muscle function in women with OAB.
Methods: In this comparative cohort study, EMG signals of patients with complaints of OAB were compared to healthy volunteers. The MAPLe probe with 24 electrodes was used for EMG registration of the different sides and depth of the pelvic floor musculature. Comparisons of individual electrodes between groups were made for the mean value of tone at rest and for every single MVC’s and endurance contractions. Linear mixed effect models were performed to analyze the relationship between groups and the EMG activity from the first to the last contractions.
Results: Sixty-five women, 50 with complaints of OAB and 15 healthy women were assessed. Average EMG signals per electrode were calculated for tone at rest, MVC, and endurance contractions. Reduction in EMG activity for OAB was found between 0.8-44.3% for tone at rest. Repeated measurements showed a significant decrease in EMG-activity for OAB between 28.7%-64.9% for MVCs and between 32.3-68.3%, endurance contractions, especially around the puborectal muscle. There was no faster decline in EMG activity from the first to the last contraction in the OAB group compared to the control group for any of the single electrodes.
Conclusions: A significant reduction of EMG activity was found in the individual muscles at different depts and sides of the pelvic floor muscle in OAB patients compared to healthy volunteers.
Keywords: Pelvic floor musculature ; Overactive bladder; Electromyography
Abbreviations: UI: Urinary incontinence; SUI: Stress urinary incontinence; OAB: Overactive bladder; PFMT: Pelvic floor muscle therapy; PFM: Pelvic floor muscle; EMG: Electromyography MVC: Maximum Voluntary Contraction
Introduction
Approximately one-quarter of all women suffer from at least one or more pelvic floor disorders [1]. Urinary incontinence (UI) represents the most common pelvic floor disorder and comprises stress urinary incontinence (SUI) and overactive bladder syndrome (OAB) [2]. According to the International Continence Society (ICS), OAB is a symptom-defined condition characterized by urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology [3]. Depending on the definition, the estimated prevalence of the combined prevalence of UI is 17,1%. As with other pelvic floor disorders, the prevalence of UI increases with age and affects up to 43.1% of women over 40 years old [2]. OAB is associated with several chronic comorbidities, significantly poorer quality of life, and depression [4].
In the treatment of female urinary incontinence, Pelvic Floor Muscle Training (PFMT) remains the first-line conservative treatment with high levels of evidence and grades of recommendation [5]. In patients with OAB, pelvic floor muscle (PFM) contractions are considered to suppress involuntary voiding through the “voluntary urinary inhibition reflex". Contractions of the puborectal muscle and external urethral sphincter are supposed to prevent internal urethral sphincter relaxation induced by the micturition reflex and result in detrusor relaxation and suppression of involuntary voiding [6]. The number, duration, intensity, and timing of the pelvic floor contractions required to inhibit a detrusor muscle contraction is not known [7]. Furthermore, the effect of PFMT to reduce OAB symptoms in women is inconclusive in the literature [8]. Data comes from small to moderate-sized trials with different outcome measures [7, 9]. In addition, studies fail to evaluate PFM changes and use poorly described and varying exercise protocols [8].
Electromyography (EMG) of the PFM is widely used to increase our understanding of pelvic floor (dys)function. Clinically, EMG is used to evaluate motor control, coordination and location of the PFM and gives the practitioner and the patient information about PFM function: the ability to contract and to relax at the time the patient wants to, and identifying whether there is an increase or decrease in activity during a particular task [10, 11].
Changes in EMG of the PFM can be related to changes in muscle performance and motor control, specially investigated in women with stress urinary incontinence [12-15]. It is also shown that after pelvic floor muscle training, EMG activity changes and this can be related to symptom reduction [16, 17]. In literature, it seems there is a relation between higher EMG activity and better pelvic floor muscle function, in particular strength [10, 11]. However, literature is scarce on this topic and even less in patients with OAB. Out of clinical experience, our hypothesis is that EMG activity not only depends on strength but also on motor control, coordination, and specific location of EMG activity of the different muscles of the pelvic floor.
The aim of this study is to gain more insight in the pelvic floor muscle function in women with OAB by determining the differences in EMG activity for each muscle of the pelvic floor muscles during contractions and relaxation in patients with OAB compared to healthy volunteers
Material and Methods
Design and participants
This is a comparative cohort study of two separate studies. In this secondary analysis, we compare EMG data of the same diagnostic EMG assessment with a validated probe(18) and a standardized assessment protocol used in both studies.
Concerning the first study, patients with complaints of OAB were recruited by advertisement to participate in a randomized control trial. Patients were included after history taking with validated questionnaires. Patients were excluded if they used any medication for their complaints of OAB, did pelvic floor exercises or bladder training in the past, had a prolapse ≥ stage 2, suffered from neurological disorders, had a medical history of invasive perineal and/or rectal surgery, or faced existing dominant SUI. Included patients were randomly assigned to an intervention and a control group by computer-generated list [16].
Regarding the second study about co-contractions of the pelvic floor, healthy pelvic floor physiotherapists without complaints were approached by mail for participation and included if able to perform a correct pelvic floor muscle contraction, which was assessed and underwent by vaginal and anal palpation and with biofeedback in various training courses. During the assessment, a correct PFM contraction was checked by an inward and upward movement of the probe [19]. Both study protocols were approved by the medical ethical committee and all participants signed written informed consent.
Procedure
In both studies, participants were placed in a comfortable supine position, with pillows placed under the knees and head and the MAPLe® probe (Novuqare Pelvic Health B.V. CE 0344)was inserted vaginally by a pelvic floor physiotherapist with a broad knowledge of pelvic floor disorders and experience in diagnostics and treatment of pelvic floor dysfunctions. The MAPLe is a probe for EMG registration of the pelvic floor musculature with a matrix of 24 electrodes enabling the measurement of EMG activity from the different sides and layers of the PFMs and a grounding electrode on the spina iliac anterior superior [18].
Participants were asked to perform three consecutive tasks, according to a standardized protocol:
One-minute rest, where participants were instructed to relax and breathe normally.
Ten maximum voluntary contractions (MVC's) where participants were verbally instructed by the researcher to perform a short controlled (maximum)contraction for one second without contracting the muscles surrounding the pelvic floor and relax the pelvic floor muscles between the MVC contractions for 3 seconds.
Three endurance contractions where participants were verbally instructed to contract the pelvic floor muscles at such a level that they could hold for 30 seconds, without contracting the muscles surrounding the pelvic floor and relax the pelvic floor muscle for 60 seconds between the endurance contractions [20, 21].
During these examinations, no instructions were given on how to perform a correct pelvic floor muscle contraction. In our opinion, giving instructions could influence the results since you are providing biofeedback on the muscle function [22]. The assessment was conducted by a researcher who held the probe in the right position and guiding the participants through the protocol. Both participants and the researcher were blinded to the screen. EMG signals were registered behind a computer by another researcher
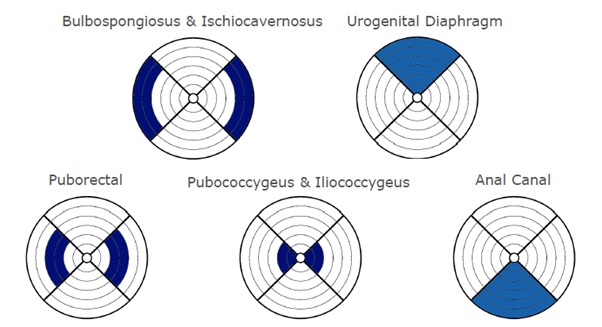
Raw EMG signals were acquired with the MAPLe system at a sample rate of 1.000 Hz; the root means square was calculated using a window of 100 samples (0.1 sec). Raw EMG data were visually controlled for artefacts. For signal analysis, mean EMG values per electrode (24 in total) were calculated for tone at rest, for every single MVC and every single endurance contraction. A graphical representation of the location of the muscles with respect to the electrodes can be found in (Figure 1).
Statistics
Baseline characteristics data were presented for age in the form of the mean and standard deviation (SD) using independent samples T-test for the differences between groups and presented for parity and postmenopausal states in frequency (percentages) using the Fischer’s exact test for differences between groups. Comparisons of individual electrodes between the groups were made for the mean EMG-value of tone at rest, using Students-T-test. The EMG-values of contractions in this research did not have a normal distribution. In order to compare these values, a logarithmic transformation was used.
Linear mixed-effect models were used to analyze the differences in EMG activity between groups and to analyze the effect of repeated contractions on the EMG activity (10 consecutive MVC’s and 3 consecutive endurance contractions). In order to properly perform this analysis an age correction for baseline, imbalances were included. The fixed effects were the consecutive number of contractions, the OAB group (with and without interaction term), and an intercept. To analyze the effect of the repeated contractions, random intercepts and slopes per subject were used. The best-fitted model was chosen using likelihood ratio tests. Homoscedasticity and normality of the residuals of the selected model were checked by visual inspection.
Logarithmic transformations transform absolute differences in EMG activity between groups into relative differences. The differences per electrode in OAB patients were thus described as a relative difference of reduction in the percentage of muscle activity compared to healthy volunteers.
From these differences, p-values were obtained. For all statistical analyses, a significance level of p< 0.05 was used. Statistical analyses were performed using R (R Core Team, 2017): A language and environment for statistical computing. R Foundation for Statistical Computing (Version 1.1.463 – © 2009-2018 RStudio, Inc.)
Results
In this study 65 women, 50 women with complaints of OAB and 15 healthy women without pelvic floor complaints, were assessed. Demographic characteristics are described in (Table 1).
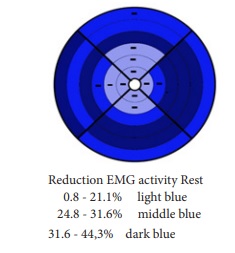
The groups were comparable for parity but not for age and menopause. EMG signals of the PFMs in patients with OAB showed a significant reduction in EMG activity compared to healthy volunteers. For tone at rest, reduction in EMG activity was found between 0.8-44.3%, especially for electrodes nearest to the urethral sphincter, the puborectal muscle on the left, dorsal and, to a lesser degree, the right side and the bulbospongiosus- and ischiocavernosus muscle on the right side (Figure 2).
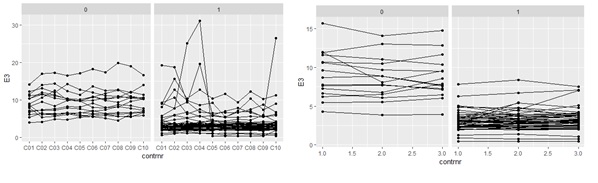
Regarding the analyses of repeated measures with linear mixed effect models, the OAB patients showed a significantly lower EMG activity for nearly every electrode from the first to the last contraction, for both the 10 consecutive MVC’s and the 3 consecutive endurance contractions compared to healthy volunteers. Within the whole study population, the EMG activity was significantly depending on age, except for the electrode nearest to the puborectal muscles. The reduction in EMG activity, as well as the consecutive contractions of the MVC’s and Endurance contractions between the two groups, was not significantly dependent on age for each electrode. Figure 3 illustrates repeated measures for one of the 24 electrodes (E3, anterior), nearest to the external urethral sphincter. Figure 4 provides an overview of the reduction in EMG activity, measured with linear mixed effect models, per electrode, and statistical significance.
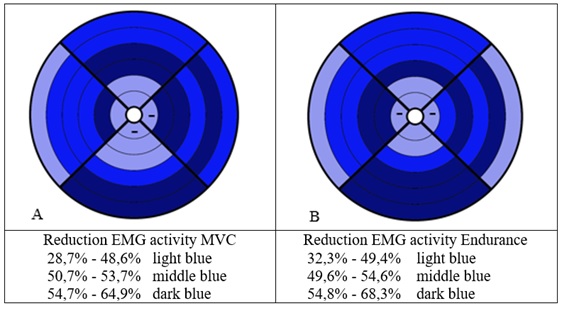
Repeated measures of MVC contractions showed a reduction in EMG activity in the OAB group compared to the control group from the first to the last contraction between 28.7-64.9%, especially for the electrodes nearest to the urethral sphincter, the pubococcygeus on both sides, the puborectal muscle on the left side, and for the bulbospongiosus- and ischiocavernosus muscle on the left side (Figure 4A). Endurance contractions showed a reduction in EMG activity between 32.3-68.3%, especially for the electrodes nearest to the urethral sphincter, to the pubococcygeus on the right side, the puborectal muscle on both sides and the bulbospongiosus- and ischiocavernosus muscle on the left side (Figure 4B). Visual inspection of residuals plots did not reveal any obvious deviations from homoscedasticity or normality
In the last step, an interaction term between the OAB group and the number of contractions was entered to examine differences in the rate of decrease (slope) of EMG activity from the first to the last contraction in groups. No significant interaction for any of the single electrodes was found, hence there is no faster decline in EMG activity from the first to the last contraction in the OAB group compared to the healthy group.
Discussion
To our knowledge, this is the first study comparing EMG signals of patients with OAB and EMG signals of healthy volunteers with a validated monopolar probe, registering EMG activity on the different sides and depths nearest to each muscle of the pelvic floor. The results of this study show a significant reduction in EMG activity of the several layers and sides of the PFM in patients with complaints of OAB compared to healthy volunteers in MVC’s and endurance contractions as well as tone at rest, especially around the puborectal muscle. The consecutive contractions, ten MVC’s, three endurance contractions, did not show a faster decline in EMG activity from the first to the last contraction in the OAB group compared to healthy volunteers.
Evaluation of muscle function in OAB patients is scarce; therefore, the mechanism of PFM dysfunction in women with OAB is not well understood nor sufficiently reviewed [8]. On the other hand, it is well known that pelvic floor complaints are caused by different layers of the pelvic floor musculature [15,16, 22, 23]. In addition to the EMG measurements of the PFM, there are no fixed normal values for rest- and contraction activity [10]. Increasing EMG activity often correlates with increasing strength, however, EMG amplitude cannot be used to quantify pelvic floor muscle strength [11, 24, 25]. The findings of this study suggest that the percentage of reduced activity explains more about the dysfunction of the PFMs in participants with OAB compared to healthy volunteers than the absolute difference of the mean EMG values. In the same way, the location of the reduced activity of the different layers of the pelvic floor muscle seems to play an important role in patients with OAB.
Some authors concluded that patients with OAB have a weak levator ani muscle, in particular the puborectal muscle and external urethral sphincter, and were unable to contract the PFM effectively to inhibit detrusor contractions [6, 22]. In line with our findings, Burgio et al. found reasons to believe that improving better control over pelvic floor muscle contraction, using biofeedback, is necessary to activate the reflex pathway and not to improve the strength of the pelvic floor muscles [26]. This seems to be confirmed by a study of the anatomical components of urinary continence by Wallner et al., who found that simultaneous contraction of the levator ani muscle and the external urethral sphincter causes an anteriorly convex bend in the mid-urethra, which closes the mid-urethral lumen. They also detected that external urethral sphincter in females is anchored to the levator ani muscle via a tendinous connection [27]. Looking at the grids in this study, the first impression is that reduced activity is located especially at the electrodes nearest to the puborectal muscle (Figures 2 and 4). The specific location of the reduction of EMG activity around the puborectal muscle (inward, upward movement) is possibly also connected to a loss of awareness, which can be considered as a loss of coordination and motor control. Furthermore, the constant relative decline in EMG activity in OAB patients implicates that these patients did not reveal indications of fatigue [6, 25, 26].
Research demonstrates that 30% of women during the first consultation with a health care professional were not able to perform a correct pelvic floor muscle contraction [24]. Neels et al. showed that 75% of the peripartum women and 68% of the postmenopausal women felt insufficiently informed about the pelvic floor function in one study [28]. In nulliparous women, a total of 93% of the women were insufficiently informed [29]. On the other hand, Vermandel et al. found that 74% of women early post-delivery improved their PFM contraction after verbal instruction [30]. In some studies, participants (either patients or volunteers without complaints) were taught to perform a correct contraction and were excluded when they were not able to do so, or they were first trained to perform a correct PFM contraction and after that included in the study [13, 31]. The authors believe that the inability to produce a correct pelvic floor muscle contraction could be the underlying cause of pelvic floor
complaints. These studies might, therefore, introduce “selection bias”, because the groups don't represent a heterogenic population where not everyone can contract the PFM. The patients with complaints of OAB, used in this research, received no instructions on how to perform a correct pelvic floor muscle contraction. It was hypothesized that not only strength but also the lack of awareness and the lack or loss of motor control in patients with OAB are a substantial part of the reduction in EMG signals [32, 33]. The specific location and the absence of a faster decline support this hypothesis. In literature, there are only a few studies investigating the changes in EMG signals in OAB patients. The limitations of EMG are that electrodes do not always correspond with the different pelvic floor muscles [34], they use bipolar configurations [35] and that probes give pre-stretching and are sensitive for crosstalk. The MAPLe probe uses a monopolar configuration, a validated location, and is not sensitive for crosstalk [18]. In the study of Knight et al. no significant difference in EMG signals were found in MVC and endurance contractions in especially OAB dry compared with asymptomatic women. However, in contrast to this study most participants (46 out of 56) were nulliparous [36]. Gunnarsson et al. specifically examined MVC’s and showed a decreased neuromuscular vaginal EMG activity in all types of incontinence, stress, urge, mixed incontinence compared to healthy volunteers, but no significant differences between the separate types of incontinence. Furthermore, they found a successive decrease in EMG activity with increasing age in all incontinence groups, highly significant in women older than 50 years. This was not seen in healthy subjects [12]. However, in this study was found that within the whole study population the EMG activity was significantly depending on age. This study has some limitations. First of all, the control group, healthy pelvic floor physiotherapists, cannot easily be generalized to healthy women without pelvic floor complaints. Secondly, the control group was small, and the groups were not menopausal-, and age-matched. However, parity was not significantly different in the groups and the reduced EMG activity between the groups did not significantly depend on age. Nevertheless, there could be an influence of parity and menopausal status. In literature, it is stated that during the female life cycle the PFMs go through several adaptive changes such as pregnancy, delivery, and changes in the hormonal state which, in combination or by itself, can cause PFM dysfunction (s). In reference to the impact of childbirth, Bothelho et al. found a significant decrease in pelvic floor muscle activity measured with EMG after vaginal delivery, which was not observed in the cesarean section group [37]. Some authors state that the role of menopause on pelvic floor dysfunction is unclear, while other authors argue that menopause is associated with the etiology of urinary incontinence and OAB [3,38]. Pereira et al. showed a negative correlation between EMG activity and age and parity [31]. Despite the fact that no significant difference in parity between groups was found in this study, parity could have influenced the results.
Menopausal status and parity could have affected the EMG signals recorded from the vaginal probe because of their distance to muscle and connective tissue. Lower EMG amplitudes recorded in the OAB group may be related to the proximity of the electrodes to the contractile tissues. However, this cannot be fully explained by the substantial differences we found in this study. Taking the limitations of this study into account, the great reduction in the specific location of EMG activity in this study is likely to be triggered by complaints of OAB and in our opinion, the reduced motor control and coordination in these women. We suggest in future research that it could be relevant to conduct comparative studies with different standardized exercise protocols to investigate the effect of exercises designed to improve motor control and coordination in this population.
Conclusion
This is the first study that compared EMG signals of patients with OAB with EMG signals of healthy volunteers, showing differences in EMG activity in different layers on the different sides and depths of the pelvic floor. Women with OAB demonstrate lower muscle activity especially nearest to the puborectal muscle during rest, MVC, and endurance.
Acknowledgment
The first author would like to thank the Department of Urology of the Leiden University Medical Center for giving her the opportunity to finish her master thesis clinical epidemiology at this Department.
- Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, et al. (2014) Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol 123:141-148.
- Dieter AA, Wilkins MF, Wu JM (2015) Epidemiological trends and future care needs for pelvic floor disorders. Curr Opin Obstet Gynecol 27: 380-384.
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 21: 5-26.
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. (2003) Prevalence and burden of overactive bladder in the United States. World journal of urology 20: 327- 336.
- Dumoulin C, Hunter KF, Moore K, Bradley CS, Burgio KL, Hagen S, et al. (2016) Conservative management for female urinary incontinence and pelvic organ prolapse review 2013: Summary of the 5th International Consultation on Incontinence. Neurourol Urodyn 35:15-20.
- Shafik A, Shafik IA (2003) Overactive bladder inhibition in response to pelvic floor muscle exercises. World journal of urology 20: 374-377.
- Dumoulin C, Cacciari LP, Hay-Smith EJC (2018) Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. The Cochrane database of systematic reviews 10:Cd005654.
- Monteiro S, Riccetto C, Araujo A, Galo L, Brito N, Botelho S (2018) Efficacy of pelvic floor muscle training in women with overactive bladder syndrome: a systematic review. Int Urogynecol J 29: 1565-1673.
- Greer JA, Smith AL, Arya LA (2012) Pelvic floor muscle training for urgency urinary incontinence in women: a systematic review. Int Urogynecol J 23: 687-697.
- Enck P, Vodusek DB (2006) Electromyography of pelvic floor muscles. J Electromyogr Kinesiol 16: 568-577.
- Vodusek DB BK, Berghmans B, Morkved S, Van Kampen M (2015) Evidence-based physical therapy for the pelvic floor: bridging science and clinical practice P19-42.
- Gunnarsson M, Mattiasson A (1999) Female stress, urge, and mixed urinary incontinence is associated with a chronic and progressive pelvic floor/vaginal neuromuscular disorder: An investigation of 317 healthy and incontinent women using vaginal surface electromyography. Neurourol Urodyn 18: 613-621.
- Burti JS, Hacad CR, Zambon JP, Polessi EA, Almeida FG (2015) Is there any difference in pelvic floor muscle performance between the continent and incontinent women? Neurourol Urodyn 34: 544-548.
- Madill SJ, Harvey MA, McLean L (2010) Women with stress urinary incontinence demonstrate motor control differences during coughing. J Electromyogr Kinesiol 20: 804-812.
- Devreese A, Staes F, Janssens L, Penninckx F, Vereecken R, De Weerdt W (2007) Incontinent women have altered pelvic floor muscle contraction patterns. J Urol 178: 558-562.
- Voorham JC, De Wachter S, Van den Bos TWL, Putter H, Lycklama ANGA, (2017) The effect of EMG biofeedback-assisted pelvic floor muscle therapy on symptoms of the overactive bladder syndrome in women: A randomized controlled trial. Neurourol Urodyn 36: 1796-1803.
- Gentilcore-Saulnier E, McLean L, Goldfinger C, Pukall CF, Chamberlain S (2010) Pelvic floor muscle assessment outcomes in women with and without provoked vestibulodynia and the impact of a physical therapy program. J Sex Med 7:1003-1022.
- Voorham-van der Zalm PJ, Voorham JC, van den Bos TW, et al. (2013) Reliability and differentiation of pelvic floor muscle electromyography measurements in healthy volunteers using a new device: the Multiple Array Probe Leiden (MAPLe). Neurourol Urodyn 32: 341-348.
- Voorham JC (2018) Pelvic floor muscle activation during contractions of the muscles surrounding the pelvic floor Neurourol Urodyn.
- Vereecken RL, Derluyn J, Verduyn H (1975) Electromyography of the perineal striated muscles during cystometry. Urologia Internationalis 30: 92-8.
- Hulten B, Thorstensson A, Sjodin B, Karlsson J (1975) Relationship between isometric endurance and fibre types in human leg muscles. Acta physiologica Scandinavica 93: 135-138.
- Kenton K, Brubaker L (2002) Relationship between levator ani contraction and motor unit activation in the urethral sphincter. American Journal of Obstetrics and Gynecology 187: 403- 406.
- Morin M, Binik YM, Bourbonnais D, Khalife S, Ouellet S, Bergeron S (2017) Heightened Pelvic Floor Muscle Tone and Altered Contractility in Women With Provoked Vestibulodynia. J Sex Med 14: 592-600.
- Bo K, Sherburn M (2005) Evaluation of female pelvic-floor muscle function and strength. Physical therapy 85: 269-282.
- Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B, et al. (2017) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J 28: 191-213.
- Burgio KL, Goode PS, Locher JL, Umlauf MG, Roth DL, Richter HE, et al. (2002) Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: a randomized controlled trial. Jama 288: 2293-2299.
- Wallner C, Dabhoiwala NF, DeRuiter MC, Lamers WH (2009) The anatomical components of urinary continence. Eur Urol 55: 932-943.
- Neels H, Tjalma WA, Wyndaele JJ, De Wachter S, Wyndaele M, Vermandel A (2016) Knowledge of the pelvic floor in menopausal women and in peripartum women. Journal of physical therapy science 28: 3020-3029.
- Neels H, Wyndaele JJ, Tjalma WA, De Wachter S, Wyndaele M, Vermandel A (2016) Knowledge of the pelvic floor in nulliparous women. Journal of physical therapy science 28: 1524-1533.
- Vermandel A, De Wachter S, Beyltjens T, D'Hondt D, Jacquemyn Y, Wyndaele JJ (2015) Pelvic floor awareness and the positive effect of verbal instructions in 958 women early postdelivery. Int Urogynecol J 26: 223-228.
- Pereira LC, Botelho S, Marques J, Adami DB, Alves FK, Palma P, et al. (2016) Electromyographic pelvic floor activity: Is there an impact during the female life cycle? Neurourol Urodyn 35: 230-234.
- Madill SJ, Pontbriand-Drolet S, Tang A, Dumoulin C (2013) Effects of PFM rehabilitation on PFM function and morphology in older women. Neurourol Urodyn 32: 1086-1095.
- Thompson JA, O'Sullivan PB, Briffa NK, Neumann P (2006) Altered muscle activation patterns in symptomatic women during pelvic floor muscle contraction and Valsalva manouevre. Neurourol Urodyn 25: 268-276.
- Voorham-van der Zalm PJ, Pelger RC, van Heeswijk-Faase IC, Elzevier HW, Ouwerkerk TJ, Verhoef J, et al. (2006) Placement of probes in electrostimulation and biofeedback training in pelvic floor dysfunction. Acta obstetricia et gynecologica Scandinavica 85: 850-855.
- Keshwani N, McLean L (2015) State of the art review: Intravaginal probes for recording electromyography from the pelvic floor muscles. Neurourol Urodyn 34:104-112.
- Knight S, Luft J, Nakagawa S, Katzman WB (2012) Comparisons of pelvic floor muscle performance, anxiety, quality of life, and life stress in women with dry overactive bladder compared with asymptomatic women. BJU Int 109: 1685-1689.
- Botelho S, Riccetto C, Herrmann V, Pereira LC, Amorim C, Palma P (2010) Impact of delivery mode on electromyographic activity of pelvic floor: a comparative prospective study. Neurourol Urodyn 29: 1258-1261.
- Mannella P, Palla G, Bellini M, Simoncini T (2013) The female pelvic floor through midlife and aging. Maturitas 76: 230- 234

Tables at a glance
Figures at a glance