Changes of Serum Complement During Normal Pregnancy and the Establishment of Reference Intervals
Received Date: April 11, 2020 Accepted Date: May 03, 2020 Published Date: May 05, 2020
doi: 10.17303/jwhg.2020.7.202
Citation: WenJing Wu (2020) Changes of Serum Complement During Normal Pregnancy and the Establishment of Reference Intervals.J Womens Health Gyn 7: 1-8.
Abstract
Objective: To detect and investigate the changes of serum complement levels, C3 and C4, in healthy pregnant women and establish their reference intervals.
Method: 434 healthy pregnant women were divided into early, middle, and late pregnant groups according to their different gestational ages. At the same time, 133 healthy and in-pregnant women were selected as the control group; the levels of serum C3 and C4 were determined by immunoturbidimetry in each group. We analyzed the statistical differences and established RIs (reference intervals) of serum complement levels during different pregnant periods.
Results: The reference interval of serum C3 level in healthy non-pregnant women was (0.554-1.055) g/L, that of C4 was (0.104-0.354) g/L. The reference interval for serum complement C3 andC4 levels in healthy pregnant women was (0.183- 1.019) g/L and (0.005-0.225) g/L, but there was no significant difference in serum complement levels between different pregnant periods.
Conclusions: The levels of serum C3 and C4 in pregnant women were lower than those in healthy non-pregnant women, but there was no significant difference in serum complement levels between different gestational ages. This article successfully established the RIs of serum C3 and C4 levels in pregnant women and non-pregnant women, providing a reference for clinical medical workers and laboratory personnel.
Keywords: Pregnancy; C3; C4; Reference Interval
Introduction
Complement is a multi-component complex that not only protects against invading pathogens but also contributes to the regulation of other host defense systems, and there is a need for effective pharmacological control of the activation process and/or its products in many diseases [1]. C3 and C4play an important role in complement initiation and amplification, so their level changes can be recognized as symbols of many complement-related diseases, inflammatory diseases and acute-phase disorders [2].
During pregnancy, childbearing women undergo a series of immune system changes to meet the needs of a normal pregnancy including immune cells and cytokines [3]. An active mechanism preventing a maternal immune response evolved and then established a cooperative status to help the success of pregnancy [4]. Teirila et al. [5] proposed that the complement system may serve as a regulator of the complex tolerance and clearance processes that are fundamental in a healthy pregnancy. Besides, previous studies demonstrated that abnormal serum complement levels are associated with many pregnancy-related disorders, such as eclampsia [6-7], systemic lupus erythematosus [8], antiphospholipid antibody syndrome and venous thrombosis [9]. A better understanding of the role of the complement system in pregnancy complications will allow a rational approach to manipulate its activation as a potential therapeutic strategy with the goal of protecting pregnancies and improving long-term outcomes for mother and child [10].
However, there are few studies investigating normal ranges of serum C3 and C4 range in pregnant women. And reference intervals of serum C3 and C4 mainly come from the normal reference population, which may not represent the real physiological state of pregnant women and lead to misdiagnosis and wrong treatments. Our study aimed to explore the changes of serum complement levels of C3 and C4 in healthy pregnant women during different gestation periods and to establish reference intervals for serum complement indicators, which may provide a reference for clinicians and laboratory technicians.
Materials and Methods
Subjects
We selected a total of 434 healthy pregnant women who underwent pregnancy tests in Second Xiangya Hospital, Changsha, Hunan, China from March 2019 to March 2020. The overall mean age of the population is 30.5±9.5. were presented for August. According to the gestational weeks, they were divided into three groups: 145 in the first-trimester group (1-13 weeks), 144 in the second-trimester group (14-27 weeks), and 145 in the third-trimester group (28 weeks). Meanwhile, 133 healthy non-pregnant women of the same period were randomly selected as the non-pregnant control group, with an average age of 29.7±9.2 years. Table 1 shows the baseline characteristics of the 567 healthy Chinese women included in this study. The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. All enrolled females signed the informed consent form. The selection of healthy pregnant and non-pregnant women in this investigation was based on the inclusion and exclusion criteria as described by the CLSI C28-A3 document [11]:
1. excessive drinking (>30 g per day) or smoking (>20 cigarettes
per day);
2. high blood pressure for longer than 3 years (either systolic
pressure ≥140 mmHg or diastolic pressure ≥90 mmHg);
3. body mass index (BMI) ≥28 kg/m2 or ≤18.5 kg/m2;
4. surgery during the previous 4 months of pregnancy or blood
transfusion or donation within 6 months;
5. a history of inherited diseases;
6. drug intake within 2 weeks or antibiotic abuse;
7. diagnosis of chronic disease, hepatic disease or endocrine disease;
8. heavy exercise or laborious work;
9. any abnormality of the heart, liver, lungs and kidney structure,
as determined using ultrasound or abnormal electrocardiogram;
10. the following exclusionary laboratory test results: triglyceride ≥2.26 mmol/L, total cholesterol ≥6.22 mmol/L, fasting blood glucose N 7.0 mmol/L, hepatitis B surface antigen, anti-hepatitis
C virus or anti-HIV positive, abnormal urinalysis, red cell count
≥5.0×1012/L or ≤3.0×1012/L, hemoglobin ≤110 g/L, white cell
count ≥12.0×109/L or ≤4.0×109/L, platelet count < 100×109/L.
The basic information of reference individuals was shown in Table 1.
C3 and C4 quantification
Venous blood (5ml) was collected between 8:00 a.m. and 10:00 a.m. after fasting for 8h; Then blood was left at room temperature for 40 to 60 minutes until it was completely coagulated; Serum was separated by centrifugation at a speed of 2500~3000rmp (approx. 1000g) for 5~10 minutes, and the collected serum samples were free of hemolysis, chyle, and jaundice. All measurements were conducted by C3 and C4 kits (Beckman Kurt company, USA) using the IMMAGE800 immune analyzer (Beckman Kurt company, USA), and the quality control was adjusted by fixed personnel.
Statistical analyses
All statistical analyses were performed by SPSS 22.0. For each group of data, the Kolmogorov-Smirnov test was used to analyze the distribution of the data. P > 0.05, indicated that the data were normally distributed, otherwise it was not normally distributed. F test was used to analyze the normally distributed data, and the homogeneity of variance was determined. P< 0.05 was considered to be the homogeneity of variance. The independent sample T-test was further used, and the difference of P< 0.01 was considered to be statistically significant. The nonparametric test U test was used to analyze the non-normal distribution data, and the difference of P< 0.01 was considered to be statistically significant. The non-parametric 95% percentile method was used to calculate the reference interval of serum complement C3 and C4, and the 95% confidence interval was calculated.
Results
Serum complement level distribution and RIs in healthy and non-pregnant women
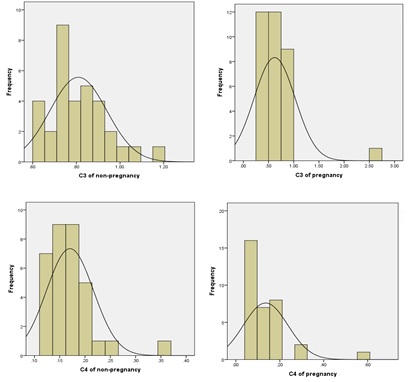
In this study, the serum complement values of healthy pregnant and non-pregnant women were tested by KomlogorovSmimov normal test, which showed that the serum complement C3 was normally distributed (P>0.05), while the serum complement C4 level was not normally distributed (Figure 1).
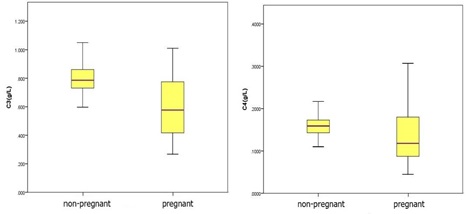
(Figure 2) demonstrated levels of serum complement C3 and C4 in the non-pregnant group and the pregnant group. The 95% reference interval for serum complement C3 levels in healthy pregnant women was (0.183-1.019) g/L and the 95% reference interval for serum complement C4 levels in healthy pregnant women was (0.005-0.225) g/L.
Distribution of serum complement levels in healthy women during early, middle and third trimesters
Table 2 demonstrated the sample measurement data during different periods of pregnancy were described by basic statistics.
Komlogorov-Smimov normality test was performed on the serum complement values of C3 and C4 in pregnant women at three different stages of pregnancy, results showed that only the serum C3 levels in the third trimester of pregnancy were normally distributed, while the serum C3 levels in the first and second trimester of pregnancy and the serum C4 levels in the first, second and third trimester of pregnancy were skewness distribution (Figure 3).
Kruskal-wallis H test showed that there was no significant difference between C3 and C4 levels of serum complement in healthy women in the first, second, and third trimester of pregnancy (P>, 0.05). Therefore, this study does not need to be divided into groups to establish reference intervals for C3 and C4 of serum complement in healthy pregnant women.
Discussion
For pregnant women, many current tests use the same reference intervals as for normal healthy adults. Furthermore, these reference intervals do not take the unique biological and biochemical characteristics of pregnant women into account. It is of great significance to establish the reference range of clinical commonly used test items based on pregnant women for improving the medical quality of pregnant women, further promoting reasonable examination and treatment, and improving the utilization efficiency of medical and health resources, which has also become an urgent problem to be solved in clinical medical laboratories. Serum complement level as an important indicator of maternal and fetal immune status during pregnancy plays an important role in the health and life safety of pregnant women and fetus, so it is essential to monitor the change of serum complement level during pregnancy.
In this study, subjects were selected strictly according to the requirements of CLSI c28-a3 document, and a reference interval of serum complement level index of healthy pregnant women was established. Serum complement levels of healthy pregnant women and healthy pregnant women were compared. Serum C3 and C4 levels of healthy pregnant women were significantly different from those of healthy pregnant women (P < 0.05), so it was particularly important to distinguish the serum complement levels of pregnant women; However, in the pregnancy group, there were no significant difference in these indexes among the early, middle and late stages of pregnancy, so we think it is not necessary to establish the reference interval of serum complement level in each stage of pregnancy. The 95% reference range of serum complement C3 level in healthy pregnant women is 0.183-1.019g/l, and the serum complement C4 level is 0.005-0.225g/l. Our results are consistent with a recent study by He et al. they also concluded that pregnancy itself may influence the plasma levels of complement system components [12].
Several basic experiments have proved the complement system was involved in the immunomodulation progress during pregnancy. Nakamura et al reported that complement may regulate the placental expression of anti-inflammatory cytokines, IL10, and TGFB1, during the latter phase of pregnancy in mice [13]. Kemp et al found that ureaplasma bacteraemia in vivo was confined to early preterm lambs with low complement function[14]. Kimura et al demonstrated that animals deficient in C3 could protect Crry knockout mice from fetal loss, while C4 or C5 deficient animals could not [15]. And Lillegard et al proved that C3a is an important product of complement activation which plays a vital role in mediating hypertension [16].
As for human, a complete complement system is essential to maintain host defense and fetal survival, because it can optimize placental development and function; Besides, complement regulation is significant at the placental interface from early pregnancy with some degree of complement activation occurring normally throughout gestation. Quantities of studies have investigated the relationship between complement and pregnancy [17]. However, few studies have established reference intervals of serum C3 and C4 during pregnancy. Complement has shown significant relation to preeclampsia. Derzsy et al found significant increases of C3a/C3 ratio and sC5b-9 in pre-eclamptic pregnancies when compared with normal pregnancies, while C3 showed a significant decrease [18]; Besides, average complement Bb levels significantly decreased over time in pregnancy with preeclampsia [19]. Oku et al proved that anti-C1q antibodies contributed to the pathogenesis of complement activation in anti-phospholipid syndrome, especially in refractory cases [20]. In an early study by Tichenor et al, up to 20% of pregnancy loss cases in the first trimester are associated with hypocomplementemia [21]. Furthermore, it was found that elevated C3a as early as the first trimester of pregnancy is an independent predictive factor for adverse pregnancy outcomes, including preterm birth, and premature rupture of the membranes. These results all highlight the significance for monitoring the change of complement during pregnancy [22].
We have successfully established reference intervals of serum C3 and C4 in pregnant women, which will provide clinicians with accurate judgment criteria to assist in the clinical evaluation of maternal/fetal immune level and diagnosis of pregnancy-related diseases.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Acknowledgments
The work was supported by the Hunan Provincial Natural Science Foundation of China (grant no.2018JJ3770).
- Lambris JD, Reid KBM, Volanakis JE (1999) The evolution, structure, biology, and pathophysiology of complement, Immunol Today20: 207-211.
- Ricklin D, Hajishengallis G, Yang K, Lambris JD (2010) Complement: a key system for immune surveillance and homeostasis, Nat Immunol 11: 785-797.
- Mor G, Cardenas I (2010) The Immune System in Pregnancy: A Unique Complexity, Am J Reprod Immunol 63: 425- 433.
- Mor G, Romero R, Aldo PB, Abrahams VM (2005) Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy, Critical reviews in immunology 25: 375-388.
- Teirila L, Heikkinen-Eloranta J, Kotimaa J, Meri S, Lokki AI (2019) Regulation of the complement system, and immunological tolerance in pregnancy, Seminars in immunology45: 101337
- Jia K, Ma L, Wu S, Yang W (2019) Serum Levels of Complement Factors C1q, Bb, and H in Normal Pregnancy and Severe Pre-Eclampsia, Medical Science Monitor 25:7087-7093.
- Larsen JB, Andersen AS, Hvas CL, Thiel S, Lassen MR, Hvas AM, Hansen AT (2019) Lectin pathway proteins of the complement system in normotensive pregnancy and preeclampsia, Am J Reprod Immunol 81.
- Kim MY, Guerra MM, Kaplowitz E, Laskin CA, Petri M, Branch DW, et al. (2018) Complement activation predicts adverse pregnancy outcomes in patients with systemic lupus erythematosus and/or antiphospholipid antibodies, Annals of the Rheumatic Diseases77: 549-555.
- Dahm AEA, Jacobsen EM, Wik HS, Jacobsen AF, Mollnes TE, Kanse SM, Sandset PM (2019) Elevated Complement C3 and C4 Levels are Associated with Postnatal PregnancyRelated Venous Thrombosis, Thrombosis and Haemostasis119: 1481-1488.
- Girardi G (2018)Complement activation, a threat to the pregnancy. Semin Immunopathol 40: 103-111.
- Clinical Laboratory and Standards Institute (2008) C28-A3 Defining, establishing, and verifying reference intervals in the clinical laboratory. Wayne, PA: CLSI.
- He YD, Xu BN, Song D, Wang YQ, Yu F, Chen Q, Zhao MH (2020) Normal range of complement components during pregnancy: A prospective study, Am J Reprod Immunol 83: e13202.
- Nakamura K, Kusama K, Bai R, Ishikawa S, Fukushima S, Suda Y, Imakawa K (2017) Increase in complement iC3b is associated with anti-inflammatory cytokine expression during late pregnancy inmice. PloS one 12.
- Kemp MW, Ahmed S, Beeton ML, Payne MS, Saito M, Miura Y, Usuda H, et al. (2017) Foetal Ureaplasma parvum bacteremia as a function of gestation-dependent complement insufficiency: Evidence from a sheep model of pregnancy. Am J Reprod Immunol 77.
- Kimura Y, Zhou L, Miwa T, Song W (2010) Genetic and therapeutic targeting of properdin in mice prevents complement-mediated tissue injury. The Journal of clinical investigation 120:3545-54.
- Lillegard KE, Loeks-Johnson AC, Opacich JW, Peterson JM, Bauer AJ, et al. (2014) Differential Effects of Complement Activation Products C3a and C5a on Cardiovascular Function in Hypertensive Pregnant Rats. J Pharmacol Exp Ther 351:344-351.
- Regal JF, Gilbert JS, Burwick RM (2015) The complement system and adverse pregnancy outcomes. Mol Immunol 67: 56-70.
- Derzsy Z, Prohaszka Z, Rigo J, Jr., Fust G, Molvarec A (2010) Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol 47:1500-1506.
- Lynch AM, Wagner BD, Giclas PC, West NA, Gibbs RS, Holers VM (2016) The Relationship of Longitudinal Levels of Complement Bb During Pregnancy with Preeclampsia. Am J Reprod Immunol 75.
- Oku K, Amengual O, Hisada R, Ohmura K, Nakagawa I, Watanabe T, et al. (2016) Autoantibodies against a complement component 1 q subcomponent contribute to complement activation and recurrent thrombosis/pregnancy morbidity in antiphospholipid syndrome. Rheumatology 55:1403-1411.
- Tichenor JR, Bledsoe LB, Opsahl MS, Cunningham DS (1995) Activation of complement in humans with a first-trimester pregnancy loss. Gynecologic and obstetric investigation 39: 79-82.
- Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, et al. (2011) Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol 117:75-83.

Tables at a glance
Figures at a glance