Cognitive Function After Treatment for Alcohol Problems
Received Date: July 04, 2021 Accepted Date: August 04, 2021 Published Date: August 06, 2021
doi: 10.17303/jnnd.2021.9.104
Citation:Sofie Have Hoffmann (2021) Cognitive Function After Treatment for Alcohol Problems. J Neurophysiol Neurol Disord 9: 1-14.
Abstract
Objective:The objective of this study was to assess cognitive function and the potential recovery in a group of patients with alcohol dependence (AD) in treatment for alcohol problems.
Methods:Patients with AD, fulfilling the ICD-10 diagnostic criteria, were recruited consecutively over a 9-months period from an outpatient clinic at Hvidovre University Hospital, Denmark. Patients’ (n=74) cognitive function was repeatedly assessed within 100 days after start of treatment by means of two methods: The quick test of cognitive speed (AQT) and continuous reaction time (CRT). The association between days from start of alcohol treatment and cognitive function was assessed in linear generalized estimating equation models, taking account of correlation structure in data and incomplete follow-up. All analyses were adjusted for sex, years of alcohol dependence, days of high alcohol use 30 days before start of treatment, other drug use, relapse, use of psychoactive medicine, and psychiatric diagnosis.
Results:At treatment start, 46 % of the patients displayed a normal score on both tests, 12 % of the patients had abnormal scores on both tests, and 42 % of the patients had an abnormal score on one test. Our results indicate a slight improvement of AQT reaction time over time, whereas the CRT test score was almost stable throughout the follow-up.
Discussion:We found no clinically significant improvement of cognitive function among patients with AD treated for alcohol problems. Further research of alcohol related cognitive impairments and recovery is needed to guide clinical practice in treatment of AD and timing of cognitive behavioral therapy.
Keywords:Treatment; Recovery; Alcohol Dependence; Cognitive Function; The Quick Test of Cognitive Speed; Continuous Reaction Time
Introduction
Alcohol is the causal factor for more than 200 diseases and conditions (Rehm, et al., 2017) [1] and is one of the leading global risk factors for the overall disease burden (World Health Organization, 2018) [2]. Studies among patients with alcohol dependence (AD) have demonstrated an approximately 3-fold higher mortality compared to the general population (Holst, Tolstrup, Sorensen, & Becker, 2017) [3]. Further, alcohol may damage multiple brain regions and a range of non-selective cognitive functions, especially if chronically consumed, causing cognitive impairments and behavioral changes (Oscar-Berman, Shagrin, Evert, & Epstein, 1997; Stavro, Pelletier, & Potvin, 2013) [4,5]. According to one study, 50 to 85 % of AD patients show signs of mild to moderate cognitive deficits (Parsons & Nixon, 1993) [6]. The degree of neuropsychological dysfunction does not correlate consistently with measures of previous alcohol consumption (George Fein, Torres, Price, & Di Sclafani, 2006) [7], but may arise from a complex interaction between the direct effects of ethanol, indirectly through damage of other organs that interferes with nerve cells in the brain, and coexisting health problems and behaviors. For example liver disease (Corrao, Bagnardi, Zambon, & La Vecchia, 2004; Udo, Vasquez, & Shaw, 2015) [8, 9], dietary deficiencies (Molina, 1994 ) [10], comorbid psychiatric disorders (Dawson, Grant, Stinson, & Chou, 2005) [11], use of psychoactive drugs (Berg & Dellasega, 1996) [12], family history of alcoholism (Drake, et al., 1995; Peterson, Finn, & Pihl, 1992) [13,14], genetics (Edenberg & Foroud, 2013; Gierski, et al., 2013) [15,16], other drug use (M. o. S. D. T. World Health Organization, 2004) [17], brain volume reduction (Ding, et al., 2004; Zahr & Pfefferbaum, 2017) [18,19] and somatic comorbidity (Lauridsen, et al., 2016) may impact cognitive function and recovery. In the most severe cases, AD patients may develop Korsakoff’s syndrome, Wernicke’s encephalopathy (Zahr & Pfefferbaum, 2017) [19] or dementia (Holst, et al., 2017) [3].
Understanding how cognitive function among AD patients develops over time is important as it may add to evidence guiding clinical practice in treatment of AD, and timing of cognitive behavioral therapy.
The aim of the present study was to the assess the association between cognitive function (measured with AQT and CRT) and time since start of treatment for alcohol problems among AD patients.
Methods
Subjects
Patients with AD were recruited consecutively over a 9-months period from an outpatient clinic at Hvidovre University Hospital, Denmark (year 2007-2008). Treatment at hospitals and hospital outpatient clinics is free of charge in Denmark, and admission is open. Patients fulfilling the ICD-10 diagnostic criteria for AD, and seeking assistance at the clinic, were invited to participate in a study evaluating nutrient intake and nutritional status, as described elsewhere (Wilkens Knudsen, et al., 2014) [23], in which cognitive function, along with other factors, were accessed.
Participation in the study was voluntary and participating patients provided informed consent that their data could be used for research. The study was approved by The Danish National Committee on Biomedical Research Ethics (ref.no. H-A-2007-0032) and the Danish Data Protection Agency (ref. no. 2007-41-0735).
Patients were excluded if they were under the age of 181, had received blood transfusion or major surgery within the last 3 months, had a malignant disease, were pregnant or breastfeeding, or if they did not understand Danish. A total of 80 patients with AD were included, comprising 29 % of all patients who entered the treatment program in the inclusion period. Included patients did not vary significantly from patients not entering the study, regarding age (p = 0.48), gender (p = 0.84), alcohol intake (p = 0.94) or duration of alcohol abuse (p = 0.97) (Wilkens Knudsen, et al., 2014).
The initial test of cognitive function was conducted as quickly as possible after treatment initiation, and hereafter patients were invited for cognitive tests approximately every 2nd week the first 2 months, and a final test 6 months after start of treatment. Six patients never showed up for the initial test, and several of the following tests were delayed or missing for some patients, as they failed to show up for their scheduled appointment or to show up at all. The final test was only conducted among 26 patients, with the last test performed 454 days after start of treatment. Patients with complete data may not be representative of AD patients in treatment, and due to potential selection bias, these test results from the final test are excluded from the analysis. This left a study population of 74 patients, with three test estimates, and 191 participant observations.
Cognitive function
Cognitive function is a complex phenomenon and consists of several domains as attention, memory, processing speed, semantic fluency, motor control, executive function, and visual-perceptual function. In this study different domains were measured by means of two tests: The Quick Test of Cognitive Speed (AQT) and Continuous reaction time (CRT).
The Quick Test of Cognitive Speed (AQT)
AQT was designed to assess perceptual and cognitive speed from visual stimuli to verbal response (Nielsen, Wiig, Warkentin, & Minthon, 2004; Wiig, et al., 2002) [24,25]. The test has been used in the evaluation of patients with Alzheimer disease (Nielsen, et al., 2004) [24] and dementia evaluation in primary care (Kvitting, Wimo, Johansson, & Marcusson, 2013) [26]. In part 1 and 2 of the test one dimension is assessed at a time, thus the patient names forms (circles, squares, triangles, or rectangles) and colors (red, black, yellow, or blue) respectively. In part 3 overall cognitive speed is assessed using both dimensions, and the patient is asked to name colors and forms of the 40 visual stimuli. The main result is extracted from part 3 of the test and measured in seconds. A test result above 70 seconds is considered abnormal. AQT has shown to be independent of language, color blindness, education, age and gender (Kvitting, et al., 2013; Takahashi, Awata, Sakuma, Inagaki, & Ijuin, 2012) [26, 27].
Continuous reaction time (CRT)
CRT is an approximately 10 minutes registration of motor reaction to audible stimuli, that measures and combines motor reactions speed, sustained attention, and inhibitory control. CRT is used for the assessment of mild forms of hepatic encephalopathy and is used as a screening tool for minimal hepatic encephalopathy in Scandinavia (Lauridsen, et al., 2016 [20]). CRT is measured by means of a set of headphones, a trigger button, a laptop, and software (Lauridsen, Thiele, Kimer, & Vilstrup, 2013) [28] (in this study EKHO was applied). The patient must press a button as fast as possible in response to each audible stimulus in the headphones. During the test, the patient is exposed to 150 randomly occurring sound stimuli (90 dB and 500 mHz) with 2 to 6 seconds intervals. The CRT index (the ratio: 50 percentile/(90 minus 10 percentile)) is a measure of intra personal reaction time stability (Lauridsen, et al., 2016) [20], and a value below 1.9 is considered abnormal (Lauridsen, et al., 2013) [28]. The CRT index has shown to be independent of gender, age and intelligence (Lally & Nettelbeck, 1977; Lauridsen, Gronbaek, Naeser, Leth, & Vilstrup, 2012) [29,30].
Covariates
Information on age, sex, highest completed education, employment status, years of alcohol dependence, other drug use, use of psychoactive medicine, psychiatric diagnosis, days of a high alcohol use (≥5 drinks/day) 30 days before start of treatment, and weekly alcohol intake 30 days before start of treatment, was obtained through systematic interviews and registered in the Copenhagen Alcohol Cohort as described elsewhere (Holst, et al., 2017) [3].
Years of alcohol dependency (<14 years/≥14 years), and days of high alcohol use (≥5 drinks per day) 30 days before start of treatment (not every day/every day) were dichotomized based on the median. Other drug use was dichotomized (yes/no), and due to the size of the study population no distinction was made between type of drug(s) used. Patients were defined as users of psychoactive medicine if they used at least one of the following products: antipsychotic medicine, antidepressants, anti-anxiety agents (benzodiazepine), anti-abstinence agents, unspecific sedatives (neuroleptic), or hypnotics (sleeping pills). Patients that were intoxicated by alcohol under the conduction of a test or in between tests were classified as having relapse (yes/no). Patients were identified as having a psychiatric diagnosis (yes/no) if they had at least one psychiatric diagnosis.
Statistical methods
The potential association between days from start of alcohol treatment and cognitive function was assessed in linear generalized estimating equation models, taking account of the correlation structure in data, where most individuals participated in multiple tests (Hanley, Negassa, Edwardes, & Forrester, 2003) [31].
Days since starts of treatment, was applied as a continuous, quadratic, and categorical variable (0-21 days, 22-35 days, 36-70 days and 71-101 days) in the models, to assess how the association was best described. To compare model fit, we utilized the QICU statistic for GEE models, which is analogous to AIC (Pan, 2001) [32]. All analyses were adjusted for sex, years of alcohol dependence, days of high alcohol use 30 days before start of treatment, other drug use, relapse, use of psychoactive medicine, and psychiatric diagnosis. Incomplete follow-ups were assumed missing-at-random, under these adjustments (see appendix 1 for characteristic of patients with incomplete follow-up). As a descriptive measure, we estimated cubic smoothing splines to the data using the smooth.spline function in R (R Core Team, 2018 ) [33].
Sensitivity analyses were conducted, to assess whether cognitive recovery varied depending on years of alcohol dependence, days of high alcohol use 30 days before start of treatment, other drug use, relapse, use of psychoactive medicine, and psychiatric diagnosis. Thus, stratified analyses were performed, and potential interactions between these factors and time since start of treatment on cognitive function were assessed.
Results
Baseline characteristics
The study population consisted of 74 patients, 70 % were men, and the average age was 49 years (table 1). The majority had a short educational level (38 %), 20 % were employed, and consumed 100 drinks per week on average in the 30 days before start of treatment. At treatment start, 46 % of the patients displayed a normal score on both tests, 12 % of the patients had abnormal scores on both tests, and 42 % of the patients had an abnormal score on one test.
The test results of AQT and CRT at baseline were weakly correlated with a correlation coefficient at -0.21 (p=0.07). Patients with abnormal values for both AQT and CRT were more likely to have a shorter education, to have been addicted to alcohol for a longer period of time, to have a higher weekly alcohol intake before start of treatment, and to experience relapse or use psychoactive medicine, than patients with normal test results for AQT and CRT at baseline.
Only few patients had biochemical signs of liver diseases (data not shown) (Wilkens Knudsen, et al., 2014).
In figure 1, the distribution of test values for AQT and CRT during the study period is illustrated. During the study period, 52 % of the patients displayed normal scores on both tests, 8 % abnormal scores on both tests, and 39 % abnormal scores on one of the tests. The test results were weakly correlated, with a correlation coefficient at -0.23 (p=0.001).
The Quick Test of Cognitive Speed (AQT) after treatment for alcohol problems
The association between time since start of treatment and AQT, adjusted for sex, years of alcohol dependence, days of high alcohol use 30 days before start of treatment, other drug use, relapse, use of psychoactive medicine, and psychiatric diagnosis is significant when time is applied as a continuous variable (p=0.002), and as a quadratic variable (p=0.03), and not when applied as categorical (p=0.09). Time as a continuous variable had the better fit (lowest QICU).
The AQT reaction time, adjusted for sex, years of alcohol dependence, days of high alcohol use 30 days before start of treatment, other drug use, relapse, use of psychoactive medicine, and psychiatric diagnosis decreased by 3.3 seconds per 30th day (β = -3.3 (95%CI: -5.4; -1.2), as presented in figure 2.
The Continuous reaction time (CRT) index after treatment for alcohol problems
The association between time since treatment start and CRT index, adjusted for sex, years of alcohol dependence, days of high alcohol use 30 days before start of treatment, other drug use, relapse, use of psychoactive medicine, and psychiatric diagnosis, is not significant when time is applied as a continuous (p= 0.19), quadratic (p=0.28), nor categorical (p=0.43) variable. However, the model with the continuous variable had the best fit (lowest QICU).
As presented in figure 3, the CRT index, adjusted for sex, years of alcohol dependence, days of high alcohol use 30 days before start of treatment, other drug use, relapse, use of psychoactive medicine, and psychiatric diagnosis, increased by 0.08 per 30th day (β = 0.08, 95%CI: -0.04; 0.20).
Sensitivity analysis
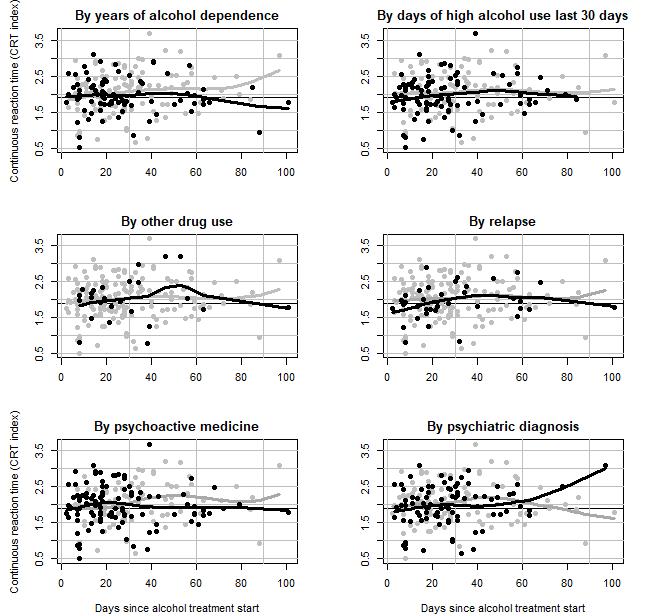
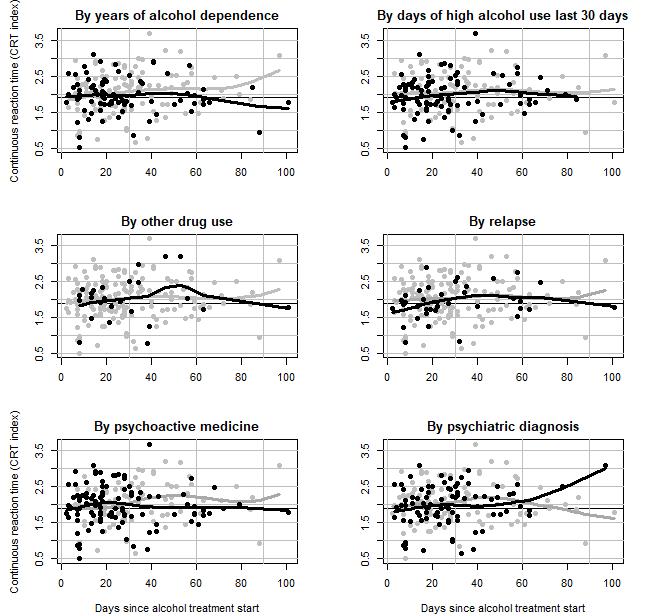
No variation was observed for the AQT test score or the CRT index by other drug use, use of psychoactive medicine, psychiatric diagnosis, days of a high alcohol use 30 days before start of treatment nor relapse (results shown in appendix 2 and 3). However, patients with less than 14 years of AD had a higher increase in CRT index per 30th day (β = 0.25, 95%CI: 0.09; 0.43), than patients with at least 14 years of AD (β = -0.05, 95%CI: -0.20; 0.09), being the only significant interaction (p=0.01).
Discussion
Our results show that 92 % of the AD patients in treatment for alcohol problems had a normal cognitive function, in at least one of the tests applied, while 54 % had an abnormal test score at either AQT or the CRT by start of treatment. The prevalence of cognitive abnormalities in our sample is relatively low compared to previous studies (Parsons & Nixon, 1993) [6]. The differences in prevalence may be related to the type of cognitive function(s) assessed and the test(s) applied. Similarly, our study demonstrated that the test scores of AQT, and CRT were weakly correlated. It is uncertain which cognitive functions that are mostly harmed by alcohol consumption, and the wide variation in the populations examined (with respect to e.g. genetic predisposition, length of AD, nutritional status and length of abstinence) has hampered researchers attempts to isolate specific brain regions and cognitive functions affected by alcohol (Zahr & Pfefferbaum, 2017) [19]. Some studies indicate that the greatest difference between AD patients and controls is found in visual modality (Glenn, Parsons, & Sinha, 1994) [34], which is essential for the AQT reaction time. While others conclude that the cerebellum part of the brain is severely affected by alcohol, resulting in loss of muscular coordination (Oscar-Berman, et al., 1997) [4], and that alcohol negatively impacts hearing (Belle, et al. 2007) [35], which may affect the CRT test score. However, a meta-analysis assessing cognitive dysfunction among alcohol dependents, including 62 studies, concluded that multiple brain regions and a range of non-selective cognitive functions are affected by alcohol, especially if chronically consumed (Stavro, et al., 2013) [22].
Further, our results indicate a slight improvement of AQT reaction time among AD patients, whereas no improvement was observed for CRT test scores, within 100 days after start of treatment for alcohol problems. The slight improvement of scores over time, are of limited clinical significance, and indicate a relatively stable status of cognitive function. Results from meta-analysis of cognitive deficits in alcoholism, suggest that significant impairments across multiple cognitive functions remain stable during the first year of abstinence from alcohol. Whereas dysfunction generally decreases by 1 year of sobriety (Stavro, et al., 2013) [22]. As thus, a longer follow-up period (than 100 days) may have revealed improvements of both AQT and CRT index.
Patients with less than 14 years of AD had a higher increase in CRT index per 30th day, than patients depended for at least 14 years. Cognitive impairments has been associated with longer duration of AD (Nowakowska, et al., 2007), less treatment compliance and fewer days of abstinence (Bates, Pawlak, Tonigan, & Buckman, 2006) [36]. Thus, patients with shorter durations of AD may be better fit to respond to treatment, which could be explanatory for these results.
The repeated measurement approach secures temporality and is a strength, however it is a limitation that the follow-up was not complete. Further, as treatment for alcohol problems is free of charge in Denmark, and admission is open, inclusion in the study is not affected by ability to pay, which may improve generalizability of results. Additionally, due to data availability we were able to adjust for several factors, potentially impacting cognitive recovery after start of alcohol treatment. However, some limitations need to be considered. Due to the incomplete follow-up and inconsistency in conduction of the tests, the analyses are based on three repeated measures conducted at different timepoints, within approximately 100 days from treatment start. Compared to previous studies, this is a relatively short follow-up period to assess cognitive recovery among AD patients. Thus, it is unknown whether the cognitive impairments are irreversible or the limited improvement in cognitive functions is a due to the relatively short period of follow-up.
As mentioned previously, cognitive impairments may arise from a complex interaction between the direct effects of ethanol, indirectly through damage of other organs that interferes with nerve cells in the brain, and coexisting health problems and behaviors. These factors may as well impact cognitive recovery. However, no information was available for the patients’ family history of alcoholism and genetics, or the prevalence of somatic comorbidity. Further, our results demonstrated that neither other drug use, use of psychoactive medicine, psychiatric diagnosis, days of a high alcohol use 30 days before start of treatment, or relapse affected either the AQT test score or the CRT index. A minority of individuals with AD seek treatment (Cohen, Feinn, Arias, & Kranzler, 2007), and AD patients that accept treatment tend to have more severe early alcohol use trajectories than untreated patients (G. Fein & Landman, 2005) [37-40], thus results may not be generalizable to untreated individuals. However, as we are interested in cognitive recovery after start of treatment, this selection bias is not of great concern.
In conclusion, we found no clinically significant improvement of cognitive function among patients with AD treated for alcohol problems. Further research of alcohol related cognitive impairments and recovery is needed to guide clinical practice in treatment of AD and timing of cognitive behavioral therapy.
Author Contributions:Sofie H. Hoffmann formulated and wrote the manuscript. Lau C. Thygesen conducted the statistical analyses and help editing and finalizing the manuscript. Anne W. Knudsen collected the majority of data. Anne W. Knudsen and Finn Zierau conceptualized the original idea of the manuscript and helped editing and finalizing the manuscript. Povl U. Becker provided significant guidance and helped edit and write the manuscript.
Conflict of Interest:The authors declare that they have no conflict of interest.
Funding:This research was funded by Copenhagen University Hospital Hvidovre
Ethical Approvals:The Danish National Committee on Biomedical Research Ethics (ref.no. H-A-2007-0032) and the Danish Data Protection Agency (ref. no. 2007-41-0735) approved the study.
- Rehm J, Gmel GE, Gmel G, Hasan OSM, Imtiaz S,, et al. (2017) The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 112: 968-1001.
- World Health Organization (2018) Global status report on alcohol and health 2018. Retrieved from Geneva.
- Holst C, Tolstrup JS, Sorensen HJ, Becker U (2017) Alcohol dependence and risk of somatic diseases and mortality: a cohort study in 19 002 men and women attending alcohol treatment. Addiction 112: 1358-66.
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C (1997) Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World 21: 65-75.
- Parsons OA, Nixon SJ (1993) Neurobehavioral Sequelae of Alcoholism. Neurologic Clinics 11: 205-18.
- Corrao G, Bagnardi V, Zambon A, La Vecchia C (2004) A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med 38: 613-9.
- Udo T, Vasquez E, Shaw BA (2015) A lifetime history of alcohol use disorder increases risk for chronic medical conditions after stable remission. Drug Alcohol Depend, 157: 68-74.
- Molina JA, F Bermejo T, del Ser FJ, Jiménez-Jiménez A, Herranz, et al. (1994) Alcoholic cognitive deterioration and nutritional deficiencies. Acta Neurologica Scandinavica 89: 384-90.
- Dawson DA, Grant BF, Stinson FS, Chou PS (2005) Psychopathology associated with drinking and alcohol use disorders in the college and general adult populations. Drug Alcohol Depend, 77: 139-50.
- Berg S, Dellasega C (1996) The use of psychoactive medications and cognitive function in older adults. J Aging Health, 8: 136-49.
- Peterson JB, Finn PR, Pihl RO (1992) Cognitive dysfunction and the inherited predisposition to alcoholism. J Stud Alcohol 53: 154-60.
- Edenberg HJ, Foroud T (2013) Genetics and alcoholism. Nature reviews. Gastroenterology & hepatology 10: 487-94.
- Gierski F, Hubsch B, Stefaniak N, Benzerouk F, Cuervo-Lombard C,, et al. (2013) Executive functions in adult offspring of alcohol-dependent probands: toward a cognitive endophenotype? Alcohol Clin Exp Res 37: E356-363.
- World Health Organization M.o. S. D. T (2004) Neuroscience of psychoactive substance use and dependence.
- Ding J, Eigenbrodt ML, Mosley TH, Hutchinson RG, Folsom AR, Harris TB,, et al. (2004) Alcohol intake and cerebral abnormalities on magnetic resonance imaging in a community-based population of middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 35: 16-21.
- Zahr NM, Pfefferbaum A (2017) Alcohol’s Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol Res 38: 183-206.
- Fein G, Torres J, Price LJ, Di Sclafani V (2006) Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism, clinical and experimental research, 30: 1538-44.
- Nowakowska K, Jablkowska K, Borkowska A (2007) Cognitive dysfunctions in patients with alcohol dependence. Psychiatr Pol 41: 693-702.
- Stavro K, Pelletier J, Potvin S (2013) Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. 18: 203-13.
- Wilkens Knudsen A, Jensen JE, Nordgaard-Lassen I, Almdal T, Kondrup J, Becker U (2014) Nutritional intake and status in persons with alcohol dependency: data from an outpatient treatment programme. Eur J Nutr 53: 1483-92.
- Nielsen NP, Wiig EH, Warkentin S, Minthon L (2004) Clinical utility of color-form naming in Alzheimer’s disease: preliminary evidence. Percept Mot Skills, 99: 1201-04.
- Wiig EH, Nielsen NP, Minthon L, McPeek D, Said K,, et al. (2002) Parietal lobe activation in rapid, automatized naming by adults. Percept Mot Skills, 94: 1230-44.
- Kvitting AS, Wimo A, Johansson MM, Marcusson J (2013) A quick test of cognitive speed (AQT): usefulness in dementia evaluations in primary care. Scand J Prim Health Care 31: 13-9.
- Takahashi F, Awata S, Sakuma N, Inagaki H, Ijuin M (2012) Reliability and validity of A Quick Test of Cognitive Speed for detecting early-stage dementia in elderly Japanese. Psychogeriatrics 12: 75-82.
- Lauridsen MM, Thiele M, Kimer N, Vilstrup H (2013) The continuous reaction times method for diagnosing, grading, and monitoring minimal/covert hepatic encephalopathy. Metab Brain Dis 28: 231-4.
- Lally M, Nettelbeck T (1977) Intelligence, reaction time, and inspection time. Am J Ment Defic, 82: 273-81.
- Lauridsen MM, Gronbaek H, Naeser EB, Leth ST, Vilstrup H (2012) Gender and age effects on the continuous reaction times method in volunteers and patients with cirrhosis. Metab Brain Dis 27: 559-565.
- Hanley JA, Negassa A, Edwardes MD, Forrester JE (2003) Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 157: 364-75.
- Pan W (2001) “Akaike’s Information Criterion in Generalized Estimating Equations.” Biometrics 57: 120–5.
- R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Glenn S, Parsons OA, Sinha R (1994) Assessment of recovery of electrophysiological and neuropsychological functions in chronic alcoholics. Biol Psychiatry 36: 443-52.
- Bates ME, Pawlak AP, Tonigan JS, Buckman JF (2006) Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 20: 241-53.
- Fein G, Landman B (2005) Treated and treatment-naive alcoholics come from different populations. Alcohol 35: 19-26.
- Lauridsen MM, Poulsen L, Rasmussen CK, Hogild M, Nielsen MK,, et al. (2016) Effects of common chronic medical conditions on psychometric tests used to diagnose minimal hepatic encephalopathy. Metab Brain Dis 31: 267-72.
- Cohen E, Feinn R, Arias A, Kranzler HR (2007) Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 86: 214-21.
- Drake AI, Butters N, Shear PK, Smith TL, Bondi M,, et al. (1995). Cognitive recovery with abstinence and its relationship to family history for alcoholism. J Stud Alcohol 56: 104-9.

Tables at a glance
Figures at a glance