Imaging and Histopathological Features of Pilocytic Astrocytoma Involving Various Locations of Central Nervous System– Series of Multiple Cases
Received Date: January 02, 2020 Accepted Date: February 02, 2021 Published Date: February 04, 2021
doi: 10.17303/jnnd.2021.9.101
Citation: Fatima Mubarak (2021) Imaging and Histopathological Features of Pilocytic Astrocytoma Involving Various Locations of Central Nervous System– Series of Multiple Cases. J Neurophysiol Neurol Disord 9: 1-7.
Abstract
Background: Pilocytic astrocytoma is low grade glial tumor which commonly occurs in pediatric population. It is rare in adult population. It is Grade 1 tumor according to 2016 world health organization classification and they generally have good prognosis.
Methodology: The cases of five patient were reviewed which were given the histopathological diagnosis of pilocytic astrocytoma, one patient was also diagnosed as the variant of pilocytic astrocytoma which is pilomyxoid astrocytoma. All of these tumors are involving different locations of central nervous system including cerebellum, fourth ventricle, suprasellar region and optic chiasm, cerebral hemisphere and brain stem in different patients.
Conclusion: Pilocytic astrocytoma has a wide spectrum of neuroradiological presentations. Besides its classical appearance as low-grade glioma, a more atypical presentation makes the diagnosis challenging. The best method to achieve the pre-operative diagnosis is the combination of morphological and non-morphological MR features as well as site base approach of this tumor.
Keywords: Pilocytic Astrocytoma; Magnetic Resonance Imaging; Pilomyxoid Astrocytoma; Intracranial Tumors; Low grade Glioma
Introduction
Pilocytic astrocytoma is the most common central nervous system (CNS) tumor in children and adolescence. It is the most common primary brain tumor in children between 5 and 14 years [1,2]. According to WHO classification, it is considered as low grade, WHO grade I CNS tumor [3]. We are going to discuss their clinical presentations, morphological features, histological features as well as their differential diagnosis with main focus on imaging feature.
Case 1
Pilomyxoid Astrocytoma of Fourth Ventricle
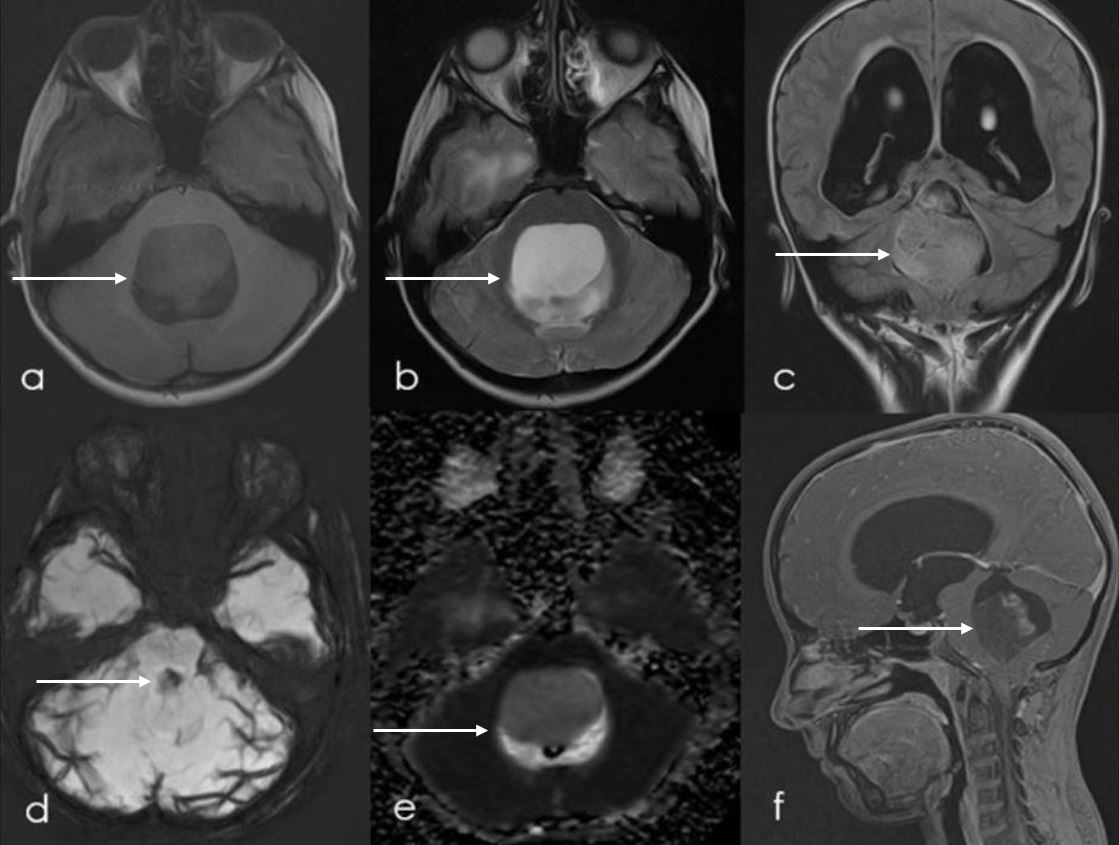
9-year-old male patient presented with complaint of disbalance, headache and vomiting for 3 months.
Magnetic Resonance Imaging (MRI) scan (Figure 1) was done which showed abnormal signal intensity space occupying lesion within floor of 4th ventricle with mass effect over brain stem and significant obstructive hydrocephalus. This lesion predominantly returns T2 hyperintense signals (Figure 1b) and hypointense signal on T1-weighted images (Figure 1a), without diffusion restriction (Figure 1e) or susceptibility focus (Figure 1d) and shows internal patchy post contrast enhancement (Figure 1f).
The patient underwent posterior fossa craniotomy and debulking of space occupying lesion. Histopathology of this lesion was performed (Figure 2) which showed monomorphic population of bipolar cells with predominant angiocentric arrangement, and prominent myxoid background (Figure 2a). It was also showing diffuse, strong immunoreactivity for Glial fibrillary acidic protein (GFAP) immunostain (Figure 2b). These features are consistent with pilomyxoid astrocytoma.
Case 2
Pilocytic Astrocytoma of Suprasellar Region and Optic Chiasm
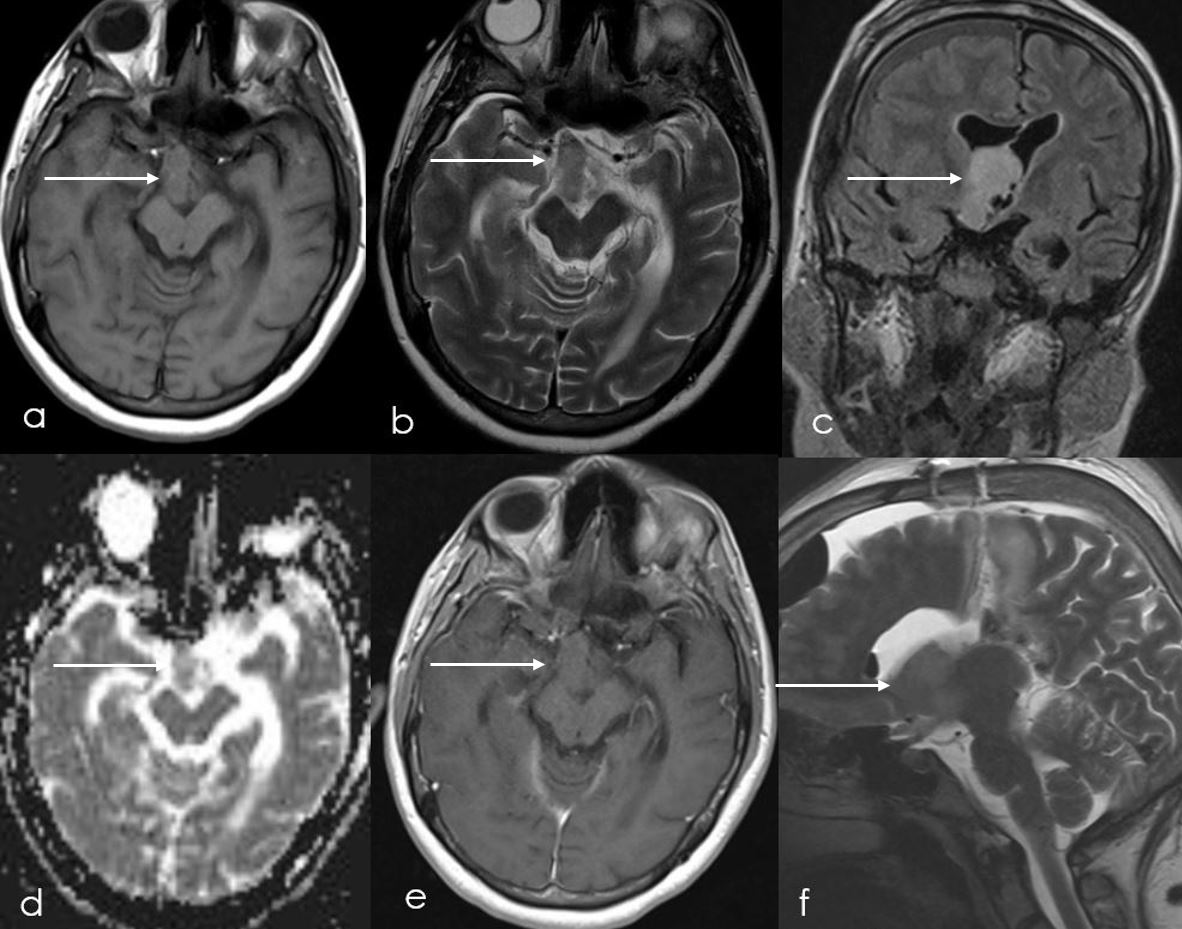
22-year-old female patient presented with complaints of drowsiness, vertigo and impaired vision for 4 months. Initially Computed tomography (CT) scan was performed (Figure 3) which showed isodense, non-enhancing lesion anterior to the third ventricle, bulging downwards towards the infundibular recess in the suprasellar area on the right side and resulting in obstructive hydrocephalus.
After that patient underwent neuronavigation guided craniotomy, excision of the lesion. Histopathology was performed (Figure 4) which showed pseudo-oligodendroglial pattern with regular round cells, rounded nuclei and thin chicken-wire vasculature (Figure 4a). Bipolar cells were showing fibrillary processes with scattered Rosenthal fibers and eosinophilic granular bodies (Figure 4b). These features are consistent with pilocytic astrocytoma.
Approximately one week after the resection, the MRI brain was performed (Figure 5) which showed residual T1 isointense (Figure 5a) and slightly T2 hyperintense (Figure 5b) non-enhancing (Figure 5e) lesion in suprasellar cistern extending into third ventricle up to the Foramen of Monroe, this residual lesion was inseparable from optic chiasm. Unfortunately, patient was lost to follow up and further could not be assessed.
Case 3
Pilocytic Astrocytoma of Cerebellum
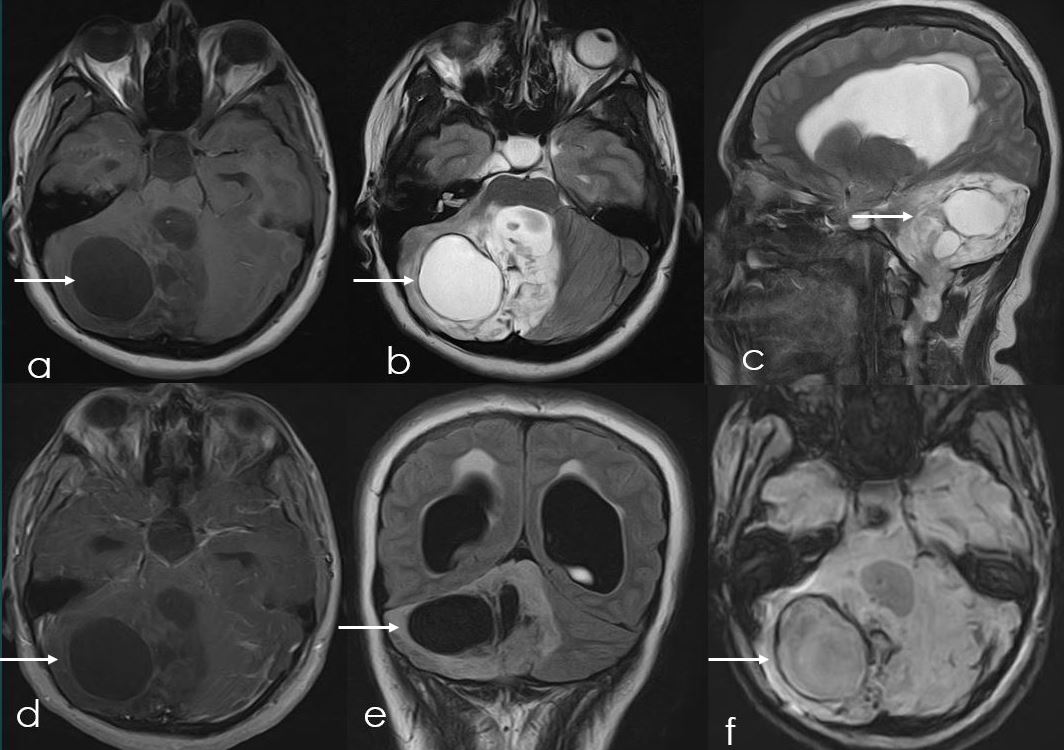
23-year-old female patient presented with complaint of headache, vomiting and difficulty in walking. The Magnetic Resonance Imaging (MRI) scan was done (Figure 6) which showed large, heterogenous solid and cystic infiltrating mass lesion in posterior intracranial fossa, involving bilateral cerebellar hemispheres, predominantly on the right side as well as involving fourth ventricle, growing along the ventricular lining inferiorly. It showed heterogenous dropout signals on susceptibility weighted images (SWI) (Figure 6f), minimal patchy post contrast enhancement (Figure 6d) and no diffusion restriction. It was resulting in gross obstructive hydrocephalus with periventricular seepage (Figure 6c).
Patient then underwent posterior fossa craniotomy and maximum safe resection of lesion. Histopathology results (Figure 7) showed bipolar cells arranged loosely in fibrillary background (Figure 7a) with long thin hair like processes (Figure 7b) as well as with focal calcification (Figure 7c). These features are consistent with pilocytic astrocytoma.
Case 4
Pilocytic Astrocytoma of Brain Stem
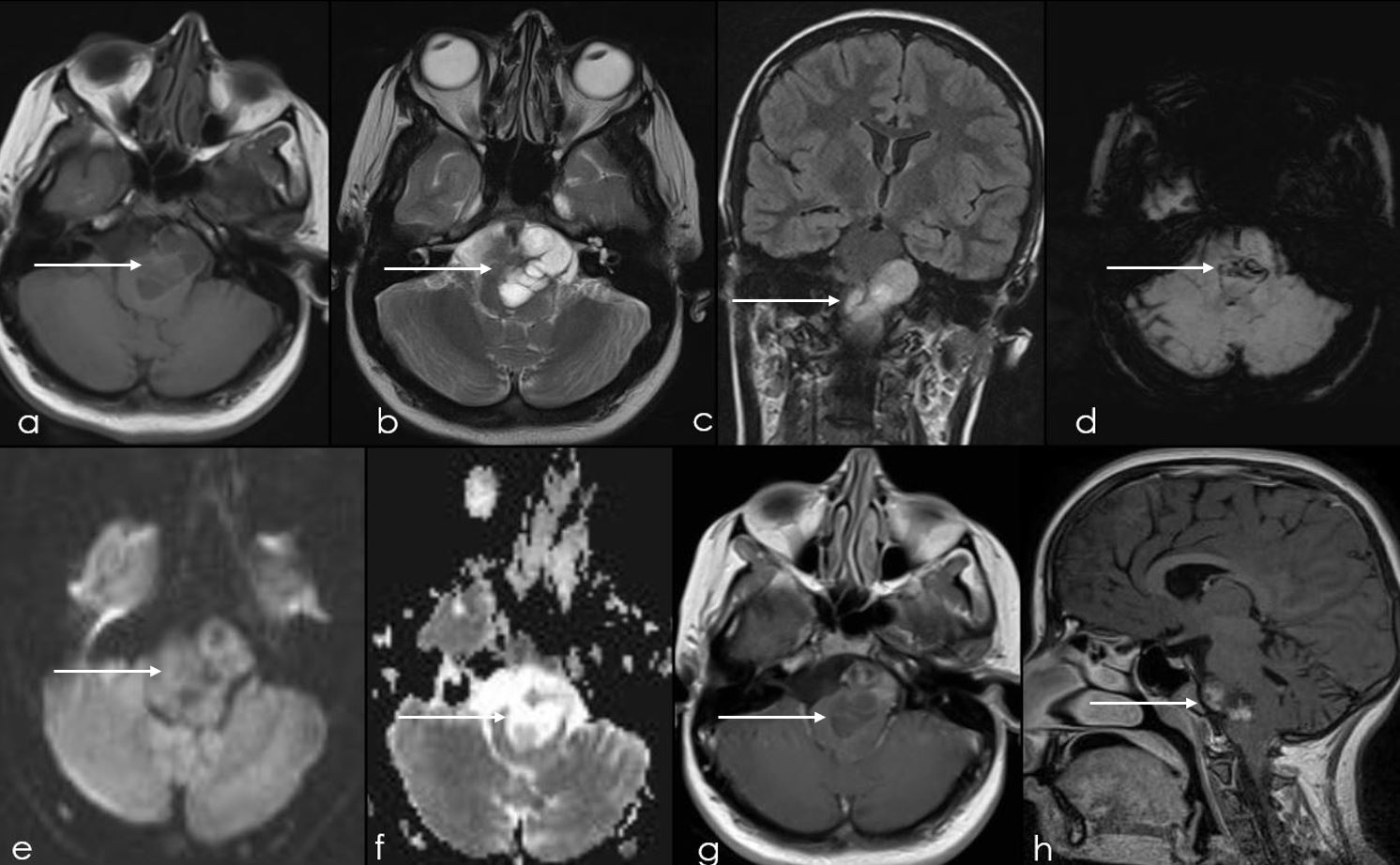
13-year-old female presented with dysarthria, dysphagia and ataxic gait. MRI brain was performed (Figure 8) which showed an irregular slightly lobulated lesion with few cystic areas noted at the pontomedullary junction. It was slightly effacing fourth ventricle without significant obstructive hydrocephalus. It was extending into the interpeduncular fossa slightly towards left of the midline. This lesion showed areas of inhomogeneous post contrast enhancement (Figure 8h), however no diffusion restriction was seen (Figure 8e). Few peripheral areas of signal dropout noted on SWI which could represent siderosis or calcification (Figure 8d). On histopathology it was proven to pilocytic astrocytoma.
Case 5
Pilocytic Astrocytoma Of Cerebral Hemisphere
16 years male presented with headache, orbital pain and vertigo for 4 days. MRI brain was performed (Figure 9) which showed a large multicystic lesion in right cerebral hemisphere involving the parietal lobe and peritrigonal region. It was extending up to the superior cerebral peduncle. It was causing compression effect on third ventricle and occipital horn of right lateral ventricle. This lesion appeared hyperintense on T2 (Figure 9b), hypointense on T1 (Figure 9a) showing peripheral enhancement (Figure 9g). There was significant perilesional edema and focal midline shift. There were foci of signal dropout within this mass with some element of layering in the dependent portion suggesting hemorrhage (Figure 9d). Overall findings suggested cystic neoplastic lesion. Patient then underwent right temporoparietal Craniotomy with excision of this space occupying lesion. On histopathology it was proven to pilocytic astrocytoma
Discussion
Demographics
Pilocytic astrocytoma is the most common central nervous system (CNS) tumor in children and adolescence and accounts for 15.5% of all cases. It is the most common primary brain tumor in children between 5 and 14 years [1,2]. Very rarely it occurs above the age of 30 and is the second most common in children between 0–4 and 15–19 years of age. According to WHO classification, it is considered as low grade, WHO grade I CNS tumor [3].
Location
Pilocytic astrocytoma can arise from anywhere though out central nervous cistern, however it most commonly arises from cerebellum, with second most common location of optic chiasm. Other less common location includes brain stem, hypothalamus, cerebral hemispheres and spinal cord [4]. In pediatric patients it is commonly seen in infratentorial region while in adults it is usually supratentorial. Pilocytic astrocytoma involving optic nerve and optic chiasm are also called optic pathway glioma (OPG), these are relatively rare and accounts for 2 to 5% of cases. 30% of OPG are associated with neurofibromatosis 1 (NF1) [5].
Clinical Presentation
Clinical presentation is variable and depends upon the site of involvement of tumor. When the tumor arises from cerebellum, it usually causes obstruction of fourth ventricle, resulting in intracranial hypertension. Symptoms includes headache, nausea/vomiting and cerebellar symptoms such as gait disturbance, slurring of speech and symptoms due to involvement of optic pathway such as blurred vision, diplopia, etc.
Imaging Features
Typical imaging features of pilocytic astrocytoma consist of well-defined cystic lesion with solid some component. The cystic component shows smooth margins with enhancing cystic wall in majority of cases. The solid portion is usually isointense to hypointense on T1 and hyperintense on T2 FLAIR images and shows post contrast enhancement [6]. Due to slow growing nature of these lesions, usually there is very little or no perilesional edema. Solid component may also show some calcification which are easily appreciated on CT and T2*/SWI images.
Although in most cases the pilocytic astrocytoma usually has cystic component but it can give atypical presentation in which pilocytic astrocytoma is completely solid without any cystic component, these are usually supratentorial in location and are usually seen in adults [7] as seen in the second case.
Histopathological Features
The typical histopathological features of pilocytic astrocytoma includes bipolar cells with fibrillary processes, scattered Rosenthal fibers and eosinophilic granular bodies. Hyalinization of blood vessels is another feature of Pilocytic Astrocytoma.
Pilomyxoid astrocytoma is a variant of pilocytic astrocytoma which is characterized by monochromatic population of bipolar cells with predominant angiocentric arrangement and prominent myxoid background, they show diffuse and strong immunoreactivity for GFAP immunostain. These tumors are more aggressive than pilocytic astrocytoma showing local recurrence and cerebrospinal fluid (CSF) seepage [8].
Treatment and Prognosis
The overall survival of patients with pilocytic astrocytoma is good in pediatric patients, with 10-year overall survival of more than 90% [2]. The survival rate decreases as the age of patient increases at the time of diagnosis. In adults, at the age of 40 years, the 10-year survival rate is closer to 70% [2]. The Overall survival rate Also depends upon the site of tumor. Tumors that can be completely resected are usually curative have better overall survival rate, for example tumors involving cerebellum and cortex than those in the brainstem and optic pathway.
Differential Diagnosis
Some of the differentials that should be considered while giving the diagnosis of pilocytic astrocytoma includes:
Hemangioblastoma: It is usually seen in adults. If it occurs in children, then it is usually associated with von Hippel-Lindau disease. Compare to pilocytic astrocytoma, the wall of cysts usually does not enhance. It usually shows smaller mural nodule with angiographic contrast blush [9].
Medulloblastoma: Most commonly occurs in younger age group (2-6 years of age). Typically arise from midline (especially vermis and roof of the fourth ventricle) while pilocytic astrocytoma usually arises from cerebellar hemisphere. They are more solid in appearance with larger heterogeneously enhancing mass [10].
Ependymoma: No recognized gender and age predilection. Usually arises from the floor of the 4th ventricle. Usually solid in nature. Tends to fill the fourth ventricle and protrude through foramen of Luschka and foramina of Magendie [11].
Cerebellar Abscess: Usually presents with symptoms of sepsis as well as symptoms of raised intracranial pressure, seizures and focal neurological deficits, well defined peripheral enhancing lesion with central restricted diffusion, associated daughter lesion and disproportionate edema is the usual presentation.
Craniopharyngioma: If the location of pilocytic astrocytoma is suprachiasmatic region then possibility of craniopharyngioma should be considered. It has bimodial distribution, first peak occurs from age of 5 to 15 and second peak occurs in adults above 40 years [12]. They are usually of mixed solid and cystic in nature with enhancing solid component. While suprachiasmatic pilocytic astrocytoma are usually solid.
Teaching Point: Pilocytic astrocytoma has a wide spectrum of neuroradiological presentations. Besides its classical appearance as low-grade glioma, a more atypical presentation makes the diagnosis challenging. The best method to achieve the pre-operative diagnosis is the combination of morphological and non-morphological MR features as well as site base approach of this tumor.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87-108.
- Lampe B, Kroll N, Piso P (2015) Prognostic Significance of Sugarbaker’s Peritoneal Cancer Index for the Operability of Ovarian Carcinoma Int J Gynecol Cancer 25: 135-44.
- Ledermann JA (2013) Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24: vi24–32.
- du Bois A, Reuss A, Pujade-Lauraine E (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-Ovar) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115: 1234Y1244.
- Luyckx M, Leblanc E, Filleron T (2012) Maximal cytoreduction in patients with FIGO stage IIIC to stage IV ovarian, fallopian, and peritoneal cancer in day-to-day practice: a retrospective French Multicentric Study Int J Gynecol Cancer 22: 1337Y1343.
- Ghisoni, E., Katsaros, D., Maggiorotto, F. et al. A predictive score for optimal cytoreduction at interval debulking surgery in epithelial ovarian cancer: a two- centers experience. J Ovarian Res 11: 42.
- Kehoe S, Hook J, Nankivell M (2013) Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: results from the MRC CHORUS trial. J Clin Oncol. 2013: 31.
- Harter P, du Bois A, Hahmann M (2006) Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 13: 1702Y1710.
- Harter P, Sehouli J (2011) Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer 21: 289Y295.
- Cavaliere F, De Simone M, Virzi S (2011) Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy:Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol 37:148Y154.
- Yonemura Y, Tsukiyama G, Miyata R (2010) Indication of peritonectomy for peritoneal dissemination. Gan To Kagaku Ryoho 37: 2306Y2311.
- Jacquet P, Sugarbaker PH (1996) Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 15: 49Y57.
- The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch Gynecol Obstet 293: 695–700.
- Prat J (2015) FIGO Committee on Gynecologic Oncology. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol 26: 87–9.
- Eisenkop SM, Spirtos NM, Friedman RL (2003) Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol 90: 390Y396.
- Fagotti A, Ferrandina G, Fanfani F (2006) A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Ann Surg Oncol 13:1156Y1161.
- Brun JL, Rouzier R, Uzan S (2008) External validation of a laparoscopic-based score to evaluate resectability of advanced ovarian cancers: clues for a simplified score. Gynecol Oncol 110:354Y359.
- Gilly FN, Carry PY, Sayag AC (1994) Regional chemotherapy (with mitomycin C) and intra-operative hyperthermia for digestive cancers with peritoneal carcinomatosis. Hepatogastroenterology 41: 124Y129.
- Japanese Research Society for Gastric Cancer (2000) The general rules for the gastric cancer study in surgery and pathology. I. Clinical classification. Jpn J Surg 11: 127Y139.
- Verwaal VJ, van Tinteren H, van Ruth S (2010) Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg 91: 739Y746.
- Koppitsch C, Sebek M (1999) Peritoneal neoplasms:scoring systems and their significance. Interdisz Onkol 4: 12Y16.
- Sugarbaker PH (1998) Current indications for cytoreductive surgery and intraperitoneal chemotherapy. In: Sugarbaker PH. Management of Peritoneal Surface Malignancy Using Intraperitoneal Chemotherapy and Cytoreductive Surgery. A Manual for Physicians and Nurses. Grand Rapids, MI: The Ludann Company: 1998.

Tables at a glance
Figures at a glance