Anxiety and Depression Following Diesel Exhaust Nano-Particles Exposure in Male and Female Mice
Received Date:February 20, 2019 Accepted Date:March 13, 2020 Published Date: March 16, 2020
doi: 10.17303/jnnd.2020.6.101
Citation: Mojtaba Ehsanifar (2020) Anxiety and Depression Following Diesel Exhaust Nano-Particles Exposure in Male and Female Mice. J Neurophysiol Neurol Disord 8: 1-8.
Abstract
Anxiety and depressive are fundamental psychic disorder and are considered one of the most severe mental health problems globally. There is much evidence that air pollution exposure is significantly related to symptoms of anxiety and depression. Air pollution exposures in addition to increased morbidity and mortality caused by cardiovascular and respiratory diseases, may cause neuroinflammation and oxidative stress and contribute to the escalating prevalence of central nervous system (CNS) diseases. Diesel exhaust particles (DEPs), is one of the most important components of air pollution. Diesel exhaust (DE) contains more than 40 toxic air pollutants and is a major constituent of ambient particulate matter (PM), particularly of ultra fine-PM.We hypothesized that females may be less susceptible than males to DEPs exposure neurotoxicity, anxiety, and depression. So adult male and female NMRI mice were exposed to DEPs (350–400 μg/m3 for 6 h per day, five days per week and 8weeks). The degree of depression by Forced Swimming Test (FST) and anxiety by elevated plus-maze test, showed an increase in male and female mice.But the observed effects were less pronounced in male than in female mice in a number of cases. Findings indicate that sub-chronic exposure to DEPs causes anxiety and depression, and suggest that gender may play important roles in modulating susceptibility to anxiety and depression-related DEPs neurotoxicity.
Keywords:Air Pollution; Diesel exhaustNano-particles; Neurotoxicity; Anxiety and Depression
Introduction
Air pollution is a mixture contained various components, including gases, particulate matter (PM), metals and organic compounds. Traffic-related air pollution is an important source of environmental pollution. It is estimated that 20 to 70 percent of environmental pollutions are combustion-derived particles of traffic [1, 2] resulting from the combustion of traffic, and 85% of PM in urban areas is related to traffic [3]. Today association between air pollution exposure and morbidity and mortality caused by cardiovascular and respiratory diseases is well established [4, 5], while new evidence suggests that air pollution may also contribute to central nervous system (CNS) diseases and negatively affect the CNS [6-8]. Epidemiological studies state that increased air pollution exposure is related to auditory and olfactory deficits, decreased cognitive functions, also increased the incidence of neurodegenerative disease pathologies and depressive symptoms [9, 10].PM is believed to be the most important threat between air pollution components and has been heavily implicated in disease [8, 11, 12]. Particulate matter is broadly determined by aerodynamic diameter (e.g. PM10 and PM 2.5). Ultrafine PM (UFPM; < 100 nm) is of very concern, as these PM can easily enter the circulation and after passing from blood-brain barrier (BBB), transmission and spread to various organs such as the brain [6, 7, 13].
One of the major reasons for global air pollution is traffic- related air pollution and the most important component is diesel exhaust (DE) [14]. DE is a complex combination of gases, hydrocarbons, sulfur, heavy metals and particulates generated within the combustion of diesel fuel [15]. Diesel exhaust gas particles (DEPs) are one of the main components of environmental particles. Most diesel exhaust gas particles have a diameter of less than 1 micron [16]. DE exposure is often the indicator of showing of traffic-related air pollution. DE is a major source of ambient PM, that contains more than 40 toxic air pollutants and particularly of UFPM. Some research to DE controlled exposure have examined on humans; for example, it has been shown to induce EEG changes in humans following acute exposure to DEPs (300 μg/m3) [17]. DEPs include many combinations that have potentially deleterious effects on the immune system[18] and brain growth [19-21]. In 2013, the International Agency for Research on Cancer (IARC) identified DEPs as a human carcinogenic group based on evidence of exposure to particulate and lung cancer1. Other human systems that are affected by diesel exhaust carcinogens contain the CNS [22, 23].
Depressive is a fundamental psychic problem and is considered one of the most severe mental health problems globally [24]. Anxiety and Depression are not only associated with decreased quality of life [25], decreased work productivity [26, 27], and physical illnesses such as cardiovascular problems but also increases the suicide rate and mortality [28, 29]. Anxiety and depression are common psychiatric illnesses with distinct interpersonal differences in symptoms, where some individuals respond fully and others show only a minor response. However, individual differences in response to stress are still heavily investigated but the gender and genetic sources of these differences in response largely remain a mystery [30].
Furthermore, recent studies support the involvement of inflammation in the brain in the pathogenesis of affective disorders and impaired cognition [31, 32]. For example, anxiety and depression in the adult male mice [6] and learning and memory disorder are associated with sub-chronic DEPs exposure [32]. DE exposure in mice has been reported to alter spatial memory and learning and locomotor activity [20, 33-35].Generally, the available evidence suggests that DEPs exposure, with primary mechanisms related to neuroinflammation and to induction of oxidative stress, is associated with noxious CNS effects. Age, sex, and genetics are among the factors that can are the most relevant effect of neurotoxic outcomes [36-38].The main aim of this study was to survey whether gender differences in anxiety and depression following DEPs exposure. Therefore, we assumed that exposure to DEPs would induce anxiety and depression in male and female mice. We used sub-chronic exposure to DEPs to evaluate how extended DEPs exposure may impact anxiety and depression. Thus, 40 NMRI male and female mice in separate cages were exposed to 350-400 μg/m3 of nanoscale (< 100 nm) DEPs for 6 h per day and five days per week for 8 weeks in a closed exposure system. Following exposure, mice underwent behavioral tests such as anxiety, depression and assessing physical abilities. Consequently, the present study was conducted to the investigation between anxiety and depression following DEPs exposure, in male and female mice.
Materials and Methods
Animals and Ethics considerations
Adult female and male NMRI mice (7-8 week-old) were used in this study, that purchased from Laboratories of animal facilities, Kashan University of medical sciences (Kaums) Kashan, Iran. All mice were housed with unlimited access to water and food and were maintained in a room with the air of humidity 35– 40% and temperature of 23 °C, and a12-h dark-light cycle (light on at 6:00 a.m.). Animals were randomly assigned to control or exposure to DEPs. All experiments on animals were carried out in the match with the National Research Council Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health and the ethics committee of Kaums approved the research stages for scientific and research objective.
DEPs collection and extraction
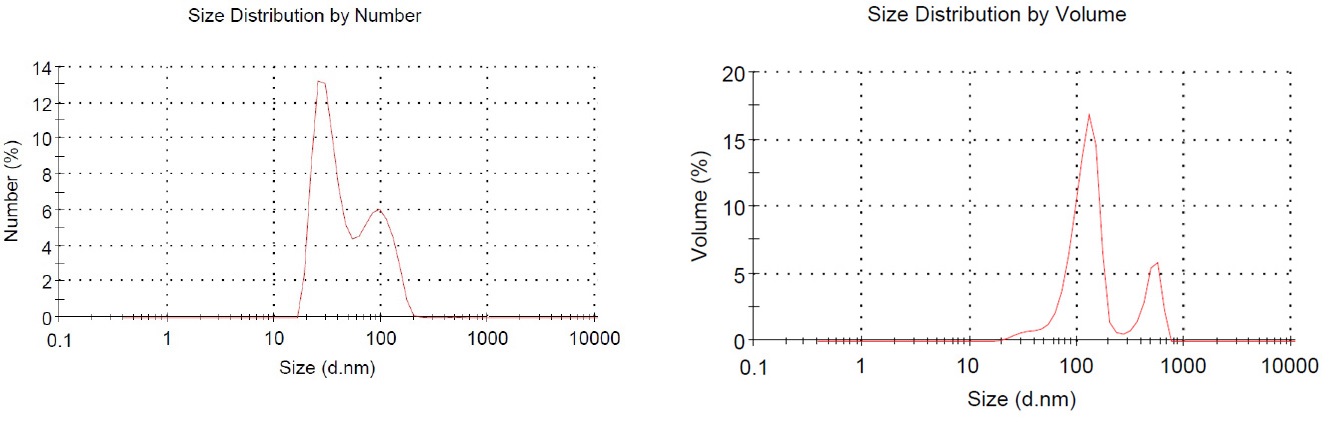
We used the following method for collected the DEPs. The engine was a light-weight (2776cc), pickup truck (Iran Khodro Diesel Co., Tehran, Iran), 4-cylinder diesel engine. The engine using standard diesel fuel at a load of 10 torques (kg/m), operated at a speed of 1,500 rpm. The exhaust gas was introduced into a stainless steel dilution tunnel (300 x 5,800 mm) at the end of the dilution tunnel. The sampling point temperature is less than 50°C. To determine the particle size distribution of DEPs in the suspension, dynamic light scattering (DLS) measurements of DEPs were performed using a Zetasizer Nano-ZS system (Malvern Instruments Ltd., UK) (Figure 1A and 1B) [6, 39].
Animals and Exposure conditions
40 NMRI 7-8 week-old female and male mice were randomly grouped (n=10 per group) and used in experiments. The mice were placed in a separate wire mesh cage (n=10) in the systemic exposure chamber (1 m3), and controlled environmental conditions (humidity, 55-60%; temperature, 21±0.5°C). The DEPs weight were measured using an electrical microbalance (readability 0.1μg) in an air-conditioned chamber (temperature, 24°C; humidity, 45%). The DEPs were resuspended in saline for 30 minutes prior to application and then vortexed for 5 minutes and sonicated for 30 minutes. Exposure of mice to DEPs was inhaled to 350-400μg DEPs/m3 for 6 h (8 AM to 2 PM)/day, 5 d/ week 8 weeks, Ultrasonic nebulizer (NE-UO7; Omron Corporation, Tokyo, Japan) with an output of 1 mL/min in a closed system room. Control mice were divided and just exposed to saline solution. Instantly after the end of the last exposure, degree of depression and anxiety by Forced Swimming Test (FST) and elevated plus maze did the measuremen.
Behavioral Experiments
Elevated plus maze
For assesses, spatial anxiety in mice used the elevated plus maze [40, 41]. The elevated plus maze device contains four 50 cm x 10 cm arms and a 10 cm x 10 cm central platform and rises 60 cm from the ground. Two opposing arms are open, and the other two arms surrounded by a 40-cm wall. As a rule, an entrance is defined as having four paws on the arm. Test room moderate lighting. While the experimenter observed the animal's behavior, each mouse was placed in a central platform facing the open arm and left to explore the maze for 5 minutes. The measured plus sign maze navigation parameters are the time in open arms (OAT) and the number of incoming open arms (OAE). The percentages of OAT and OAE are calculated as follows:
OAT%= duration spent in open arms (s) /300 (s) × 100. OAE%= number of entries into the open arms / total number of entries×100
Forced Swimming Test (FST)
FST in mice is a behavioral despair test established by Porsolt et al., previously [42]. The male and female mice were separately positioned in glass cylinders (0.15 m diameter and 0.25 m height), which encompassed 17 cm depth of 25 ℃ water. Five minutes later, removing the mice and drying and returning to their cages was performed. After 24 hours, the mice were replaced in the cylinder to measure the immobility duration for 5 minutes after the primary minute of the duration of adjustment. When mice were floating motionless, they were considered to be immobile.
Statistical analysis
Behavioral Experiment results were analyzed by oneway ANOVA with Bonferroni correction for multiple comparisons. All data are presented in the form of means ± standard error. The difference was considered the significant Alpha level at P < 0.05.
Results
Adult female and male mice exposed to 350–400 μg/ m3 DEPs for 6 h per day, 5 days per week and 8 weeks, did not significant differences between control mice and DEPs exposure mice in body length (P = 0.089), body weights (P = 0.076), eye appearance, vibrissae, and sensorimotor responses (p<0.05).
Behavioral Experiments
Anxiety
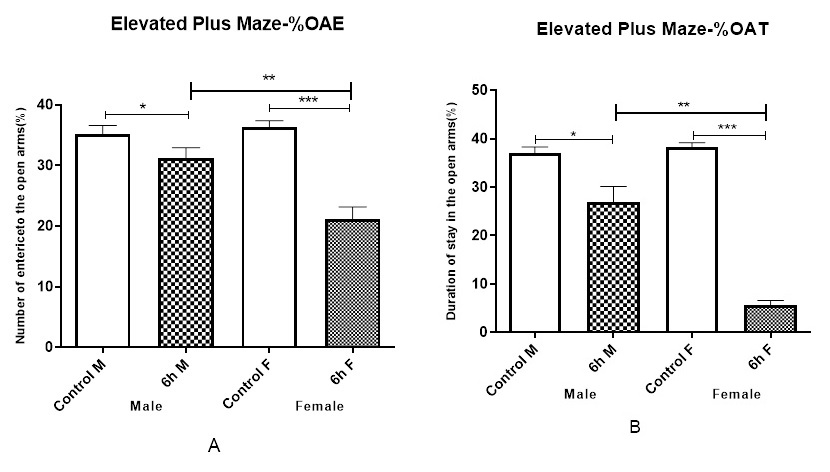
The elevated plus maze test was presented to assess the stress level of the mice exposed to DEPs. Generally, the stress level was calculated based on two factors of entering the open arms and time spent in the arms. Results show that there was a difference between the groups in terms of performance. The male and female mice exposed to 6 h/day DEPs displayed a significant decrease in the ability to enter the open arms, compared to the samples in the control group, but it was more pronounced in female than in male mice (Figure 2A). Also, the male and female mice in the 6 h exposure groups passed a shorter time in the open arms, and this difference was more in female than in male mice (Figure 2B) (p< 0.001). In the elevated plus maze decreased the number of open arm entries, and a reduced time spent in the open arms indicated reduced anxiety responses.
Depression
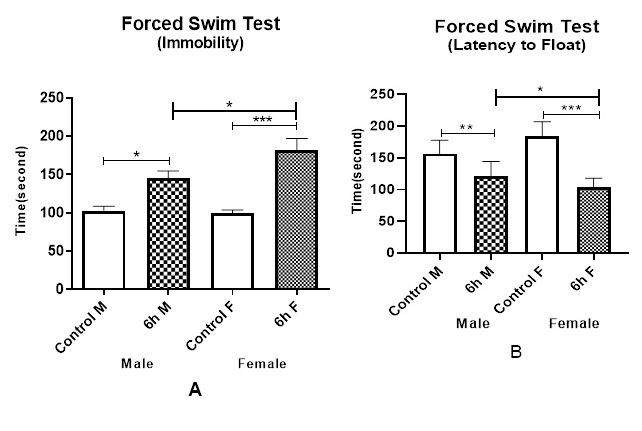
Exposure to DEPs by inducing immobility in the FST did sufficiently depressive-like responses. Sub-chronic DEPs exposure mice, with elevated duration and floating frequency in the FST, increase depressive-like behaviors. We studied the effect on immobility duration and floating frequency in forced to swim models. Based on our observations, exposure to DEPs for 6 h/ day, in male and female mice, caused significant enhancement of elevated depressive behaviors with elevated immobility duration and floating frequency in the FST (Figure 3 A) (p< 0.001). Furthermore, DEPs exposure mice had a lower latency than control mice to first float, especially in female mice, (Figure 3 B) demonstrating they more rapidly reached a state of behavioral despair than male mice (p< 0.001).
Discussion
DEPs are byproducts of fossil-fuel combustion, generally with a diameter below 100 nm. These airborne PM mimic the physicochemical characteristics of nanoparticles, demonstrating greater CNS penetrance than that of larger particles. We had reported that 14 weeks of exposure to DEPs leads to the upregulation of Oxidative stress and inflammatory markers and neuronal morphology changes in hippocampal structure resulting in anxiety and depression affective responses in male and female mice. The cognitive changes to be associated with neuroinflammation and oxidative stress in some brain regions are proved. We had previously shown that long term exposures to DEPs caused an altered behavioral in adult male mice [6]. In the one study, it was found that the risk of a depressive episode increase with arises concentration of 10 μg/m3 of PM2.5, especially in people who suffer from chronic diseases [43]. Another study has shown, that an increase in PM10 may lead to symptoms of depression in the elderly, which is more commonly associated with emotional symptoms [44]. It has been shown that during periods of highest concentrations of atmospheric particles matter, the number of suicide attempts is increasing [45]. The mechanism of this phenomenon is still experimental. In the investigations that the authors sought to find a relationship between exposure time and suicides, it was found that PM10 particles (with an aerodynamic mean ≤ 10 μm) had the greatest effect 0 to 2 days before the suicide and PM2.5 one day before they commit suicide [43].
The present study assessed the impact of DEPs exposure in female and male mice on depression and anxiety. We showed that After 8 weeks of DEPs inhalation exposure caused affective disorder in the behavioral performances on female and male mice. The mice in the exposure groups had lower activity and efficiency, compared to the control mice, whether in forced swimming test and plus-maze test. It concluded that the anxious male and female mice in forced swim test did sufficiently induce immobility duration. In addition, staying in the arms for a shorter duration was interpreted as the stress and fewer abilities to enter the open arms in the plus-maze test in male and female mice. In female mice, there was observed more anxiety and depression than male mice. In the comparison of gender effects, It was observed that the female mice responded to the forced swim test with a longer duration of immobility than male mice (Figure 2 and Figure 3). This finding provided confirmation that exposure to DEPs causes anxiety and depression [46] and suggests that gender determinants may influence these neurotoxic effects so that this gender difference in basal immobility, agrees with previous studies [47].
Exposure to PM is implicated in potentiating asthma and other cardiopulmonary situation, and behavioral despair and negative mood are associated with high airway inflammation and asthma [48, 49]. Also, air pollution exposure is directly related to emergency department visits for suicide attempts [45]. This survey is that the effects of DEPs exposure were evaluated in female and male mice and show that depression disproportionately affects women [50, 51]. DEPs exposure male and female mice increased anxiety responses compared with control mice. Male and female mice exposure to DEPs, compared with control mice, reduced the percentage of performance and activity in the center of an open field which is generally defined as (Figure 2 A, B) elevated anxiety responses [6]. Therefore, there were significant differences between DEPs exposure female and male mice than control mice in the elevated plus maze but the anxiety was lower in male mice than in female mice (Figure 2 A, B).According to epidemiologic surveys, females predominance to generalized anxiety disorder, major depression, and panic disorder, thus perhaps this gender difference is important [52-54]. These experiments also highlight the importance of gender differences in anxiety and depression models. Many articles have suggested the associated between air pollution and the prevalence of depression and suicide disorders. As we have learned, their causes are very complex, as well as the health effects of many of the subpositions in the air. While epidemiology indicates an increased risk of depression and suicide in exposed air, further research is still needed to fully explain these findings. Epidemiological data may indicate specific dependence, but further clinical and experimental studies are needed to better understand the impact of air pollution on mental health.
Conclusion
In conclusion, long-term exposure to DEPs has been shown to induce behavioral and neurotoxic effects in mice. The sub-chronic exposure to DEPs is sufficient for elicit significant increases in neurobehavior disorder, and females appear to be more susceptible to some of these effects. These findings emphasis the gender difference and importance of considering both sexes when survey neurotoxicity, and may increase susceptibility to neurotoxic effects of air pollution exposure. Further research should be examining another’s variables such as genetic and or age and exposure to DEPs impairs cognition, provokes anxiety and depressive-like behaviors in mice.
Acknowledgments
This work was supported by Genetic and Environmental Adventures Research Center, School of Abarkouh Paramedicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran; and Physiology Research Center of Kashan University of Medical sciences, Kashan, Iran.
Availability of data and materials
The dataset used in this study is available with the authors and can be made available upon request.
- Geller MD, et al. (2005) Measurements of particle number and mass concentrations and size distributions in a tunnel environment. Environmental Science & Technology 39: 8653-8663.
- Lanki T, et al. (2006) Can we identify sources of fine particles responsible for exercise-induced ischemia on days with elevated air pollution? The ULTRA study. Environmental Health Perspectives 114: 655.
- Jonidi Jafari A and M Ehsanifar (2016) The Share of Different Vehicles in Air Pollutant Emission in Tehran, Using 2013 Traffic Information. Caspian Journal of Health Research 2: 28-36.
- Rao X, et al. (2018) Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxidants & redox signaling 28: 797-818.
- Gill EA, et al. (2011) Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Progress in cardiovascular diseases 53: 353-360.
- Ehsanifar M, et al. (2019) Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicology and Environmental Safety 168: 338-347.
- Genc S, et al. (2012) The adverse effects of air pollution on the nervous system. Journal of Toxicology.
- Costa LG, et al. (2014) Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. BioMed research international.
- Weuve J, et al. (2012) Exposure to particulate air pollution and cognitive decline in older women. Archives of internal medicine 17: 219-227.
- Guxens M and J Sunyer (2012) A review of epidemiological studies on neuropsychological effects of air pollution. Swiss Med Wkly 14: w13322.
- Møller P, et al. (2010) Role of oxidative damage in toxicity of particulates. Free radical research 44: 1-46.
- Brook RD, et al. (2010) Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331-2378.
- Oberdörster G, et al. (2004) Translocation of inhaled ultrafine particles to the brain. Inhalation toxicology 16: 437-445.
- Ghio AJ, CB Smith, and MC Madden (2012) Diesel exhaust particles and airway inflammation. Current opinion in pulmonary medicine 18: 144-150.
- Hesterberg TW, et al. (2011) Particulate matter in new technology diesel exhaust (NTDE) is quantitatively and qualitatively very different from that found in traditional diesel exhaust (TDE). Journal of the Air & Waste Management Association 61: 894-913.
- Kittelson D (2007) On-road particles-characteristics and measurement. in International Workshop on Vehicle-related Nanoparticles and Environmental Health.
- Crüts B, et al. (2008) Exposure to diesel exhaust induces changes in EEG in human volunteers. Particle and fibre toxicology 5: 4.
- Takano H, et al. (2002) Lung expression of cytochrome P450 1A1 as a possible biomarker of exposure to diesel exhaust particles. Archives of toxicology 76: 146-151.
- Fujimaki H, et al. (2006) Distinct requirements for interleukin- 6 in airway inflammation induced by diesel exhaust in mice. Immunopharmacology and immunotoxicology 28: 703-714.
- Suzuki T, et al. (2010) In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Particle and fibre toxicology 7: 7.
- (2013) Cancer, I.A.f.R.o. and W.H. Organization, IARC: Outdoor air pollution a leading environmental cause of cancer deaths. No. 221. World Health Organization.
- Danysh HE, et al. (2015) Traffic‐related air pollution and the incidence of childhood central nervous system tumors: Texas, 2001–2009. Pediatric blood & cancer 62: 1572-1578.
- Filippini T, et al. (2015) A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. Journal of Environmental Science and Health, Pa 36-66.
- Adjaye-Gbewonyo K, et al. (2018) High social trust associated with increased depressive symptoms in a longitudinal South African sample. Social Science & Medicine 197: 127-135.
- Ruo B, et al. (2003) Depressive symptoms and health-related quality of life: the Heart and Soul Study. Jama 290: 215-221.
- Stewart WF, et al. (2003) Cost of lost productive work time among US workers with depression. Jama 289: 3135-3144.
- Ekman M, et al. (2013) The societal cost of depression: evidence from 10,000 Swedish patients in psychiatric care. Journal of affective disorderss 150: 790-797.
- Rumsfeld JS and PM Ho (2005) Depression and cardiovascular disease: a call for recognition. Am Heart Assoc.
- Hare DL, et al. (2014) Depression and cardiovascular disease: a clinical review. European heart journal 35: 1365-1372.
- Anisman H and RM Zacharko (1992) Depression as a consequence of inadequate neurochemical adaptation in response to stressors. The British Journal of Psychiatry 160: 36-43.
- Raison CL, L Capuron, and AH Miller (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in immunology 27: 24-31.
- Ehsanifar M, et al. (2019) Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicology and Environmental Safety 176: 34-41.
- Hougaard KS, et al. (2009) Diesel exhaust particles: effects on neurofunction in female mice. Basic & clinical pharmacology & toxicology 105: 139-143.
- Yokota S, et al. (2009) Effect of prenatal exposure to diesel exhaust on dopaminergic system in mice. Neuroscience letters 449: 38-41.
- Win-Shwe TT, et al. (2014) Effects of diesel engine exhaust origin secondary organic aerosols on novel object recognition ability and maternal behavior in BALB/c mice. International journal of environmental research and public health 11: 11286- 11307.
- Costa LG, et al. (2004) Developmental neuropathology of environmental agents. Annu. Rev. Pharmacol. Toxicol 44: 87-110.
- Tiffany-Castiglioni E, V Venkatraj and Y Qian (2005) Genetic polymorphisms and mechanisms of neurotoxicity: overview. Neurotoxicology 26: 641-649.
- Weiss B (2011) Same sex, no sex, and unaware sex in neurotoxicology. Neurotoxicology 32: 509-517.
- Yoshizaki K, et al. (2010) Subchronic effects of nasally instilled diesel exhaust particulates on the nasal and airway epithelia in mice. Inhalation Toxicology 22: 610-617.
- Carobrez A and L Bertoglio (2005) Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience & Biobehavioral Reviews 29: 1193- 1205.
- Haller J and M Alicki (2012) Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Current opinion in psychiatry 25: 59-64.
- Porsolt R, A Bertin and M Jalfre (1997) Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de therapie 229: 327-336.
- Kim Y, et al. (2015) Association between air pollution and suicide in South Korea: a nationwide study. PloS one 10.
- Lim, YH, et al. (2012) Air pollution and symptoms of depression in elderly adults. Environmental health perspectives, 120: 1023-1028.
- Szyszkowicz M, et al. (2010) Air pollution and emergency department visits for suicide attempts in Vancouver, Canada. Environmental health insights 4: EHI. S5662.
- Costa LG, et al. (2017) Neurotoxicity of traffic-related air pollution. Neurotoxicology 59: 133-139.
- Bernardi M, et al. (1989) Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiology & behavior 45: 1067-1068.
- Kullowatz A, et al. (2008) Stress effects on lung function in asthma are mediated by changes in airway inflammation. Psychosomatic medicine 70: 468-475.
- de Miguel Díez J, et al. (2011) Psychiatric comorbidity in asthma patients. Associated factors. Journal of Asthma 48: 253- 258.
- Beery AK and I Zucker (2011) Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews 35: 565-572.
- Kessler RC, et al. (1993) Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. Journal of affective disorders 29: 85-96.
- Weissman MM, et al. (1997) The cross-national epidemiology of panic disorder. Archives of General Psychiatry 54: 305-309.
- Weissman MM and M Olfson (1995) Depression in women: implications for health care research. Science 269: 799-801.
- Salvi A, et al. (2017) Psychological impact of vehicle exhaust exposure: insights from an animal model. Scientific reports 7: 1-8.

Figures at a glance