CD34+ Very Small Embryonic-like Stem Cells or Induced Pluripotent Stem Cells for Cardiac Repair?
Received Date: November 27, 2020 Accepted Date: December 28, 2020 Published Date: December 30, 2020
doi: 10.17303/jcvm.2020.6.303
Citation: Philippe Hénon, Rachid Lahlil, Anne Aries (2020) CD34+ Very Small Embryonic-like Stem Cells or Induced Pluripotent Stem Cells for Cardiac Repair?. J Cardio Vasc Med 6: 1-12.
Abstract
Current research in regenerative medicine is focused on finding pluripotent or multipotent cells with lower associated risks and fewer ethical problems when used as a treatment for patients. There are unresolved ethical and technical issues that hamper the clinical use of embryonic stem cells (ESCs). Even though induced pluripotent stem cells (iPSCs) avoid ethical issues, they present the same technical issues as ESCs, mainly related to their genomic dysregulation and epigenetic instability. In parallel, evidence has accumulated that adult bone marrow harbors different primitive cells which possess the ability to repair organs outside the hematopoietic system. It has been observed that in myocardial infarctions (MI), the injection of CD34+ cells close to the injured myocardial area can achieve a significant restoration of cardiac function, suggesting their direct and/or indirect involvement in the regeneration of heart tissue. This thought was sustained when pluripotent cells known as VSELs, which are able to differentiate and regenerate different organic tissues, were identified and isolated among CD34+ stem cells; this dismissed the presumption of the possible cell plasticity or de-differentiation. However, additional clinical studies are still to be carried out to further examine this. In the present review, we focused on the biological aspects of different stem cells to shed light on which most are promising, in the hope of improving the treatment of MI by regenerative medicine, once their manipulation becomes mastered.
Keywords: CD34+; VSELS; iPSCs; Regenerative Medicine; Acquired Heart Diseases; Cell Therapy; Myocardial Regeneration; Stem Cells; Heart Failure; Myocardial Infarction; Cardiovascular Disease
Introduction
The concept of regenerative medicine was conceived at the beginning of the 21st century, although hematopoietic stem cell transplantations-now included within this appellation-had already been performed for three decades to treat leukemias and other cancers. The goal of this new therapeutic approach is to inject stem/progenitor cells into diseased or damaged organs with the hope that they will form new healthy tissue in these organs, avoiding the need for further transplantation.
For a long time, embryonic stem cells (ESCs), which are immortalized pluripotent stem cells derived from an early embryo at the blastula stage, have been considered the ideal stem cells for applications in regenerative medicine, as they can give rise to cells from the three germ layers [1,2]. Their pluripotent differentiation potential theoretically enables them to generate any multipotent cell type, at least in vitro [3]. However, there are many problems associated with their use for clinical purposes. First, they are usually obtained by the destruction of unused embryos issued from parental projects, which is ethically highly controversial [4]. Besides ethical considerations, which reduces the possibility of their clinical use in most countries, their clinical use faces unresolved technical problems related to difficulties of achieving differentiation into fully functional cells at a large scale, the risk of teratoma or other tumor formation induced by these cells, and their genomic instability [5-7]. Furthermore, they give rise to immunogenic differentiated cells that might be rejected by a histo-incompatible recipient, requiring an immuno-suppressive treatment [8]. For these reasons, very few clinical trials using embryonic-derived progenitor cells have been authorized around the world, and most have achieved disappointing results. In the cardiology field, one group surgically sutured a fibrin patch bearing ESC-derived cardiovascular progenitors directly onto the epicardium of the ischemic myocardial area, in six patients scheduled for coronary artery bypass grafting (CABG) [9]. However, the confounding effect of the concomitant CABG, associated to the small sample size, precluded any meaningful conclusion regarding efficacy. Moreover, the intraoperative application of the patch and the alloimmunization, though clinically silent, developed by half of the patients, are limiting factors making this technology inappropriate for large scale clinical use. The therapeutic use of ESCs can now be considered to have clearly failed, despite the hype created by the media.
Over the same period of time, the successful application of hematopoietic stem cells (HSCs) in hematopoietic transplantation has encouraged clinicians to test, often empirically, adult stem cells in other therapeutic settings, mostly related to cardiac diseases [10]. In this indication, various types of cells have been employed, mainly isolated from bone marrow (BM), from peripheral blood (PB), or from fat tissue or cardiac biopsies. However, the cells used in most clinical trials performed during the two last decades were wrongly termed stem cells. For example, bone marrow mononuclear cells (BM-MNCs) are not stem cells but a mix of various cells of hematopoietic lineages at various stages of maturation, and their use in many clinical trials has failed to induce significant cardiac repair [11]. Mesenchymal stem cells (MSCs) are mainly stromal cell progenitor cells that do not have the properties required for clonal growth [12]; it is now well known that their weak beneficial effect is mainly due to the release of soluble factors or exosomes, is transient and does not induce myocardial tissue regeneration [13]. Adipose progenitor cells (APCs) issued from fat tissue are a variety of mesenchymal cells with similar properties, of which the only advantage is easy accessibility. The existence of cardiac stem cells, isolated from cardiac biopsies, is highly controversial [14]. Thus, CD34+ cells emerge as being the only “true” stem cell located in the BM.
CD34+ Cells
CD34+ cells were identified for the first time by Civin in 1984 [15]. CD34 is a membrane glycophosphoprotein that was discovered as a result of a strategy to develop antibodies that specifically recognize small subsets of human marrow cells, but not mature blood or lymphoid cells [16]. CD34 antibodies specifically detect approximately 1% of low density mononuclear cells from BM aspirates of normal donors, when there is less than 0.1% CD34 labeling of PB cells [17]. However, the intensity of CD34 expression on the cell’s membrane is heterogeneous and correlates with the stage of cell immaturity/maturity, subdividing cells into CD34bright or CD34dim subgroups. The CD34bright are smaller and less dense than the CD34dim and correspond to the earliest progenitors, i.e., stem cells, representing at best one fifth of the total CD34+ cells, while the CD34dim are larger and denser and correspond to more committed progenitor cells, having lost their clonal growth properties [18]. CD34+ cells were considered for a long time as being only HSCs, giving rise to all hematopoietic lineages [16]. As they can be mobilized in large amounts from the bone marrow into the PB by hematopoietic growth factors (HGF), achieving approximately a two-logs cell enrichment, practice of PB-stem cell (PBSC) transplantation increased exponentially from the first attempts in the mid-1980s [19,20] up to totally replacing BM transplantation in hematological cancers [21].
From the mid -1990s, it was demonstrated that “true” HSCs, inducing and sustaining post-aplasia hematopoietic recovery in the long term, and characterized by a lack of the CD38 differentiation marker [17,22], only represent a small part (≈1%) of the total CD34+ cells, raising the question of what the remaining CD34bright cells were. In 1997, Asahara et al. isolated a subpopulation of progenitor endothelial cells bearing the CD34 antigen in mice BM, which was capable of inducing neo-angiogenesis, thus demonstrating for the first time the existence of non-hematopoitic CD34+ cells [23]. In the following years it was progressively established that the CD34 antigen was also a marker of cardiac, liver, and osteoblastic progenitor cells [24-26]; each of these CD34+ subpopulations represented approximately 1% of the total CD34+ cells. In an attempt to explain this variety of CD34 subpopulations, several investigators proposed the hypothesis that HSCs could transdifferentiate into other lineages of progenitor cells, even those normally issued from another germ layer, opening the path for the transgressive concept of “cell plasticity” that persisted for years.
Apart from hematopoietic transplantation, CD34+ cells have been mainly employed in attempts to achieve cardiac repair after myocardial infarction (MI). Experimental and physiological data support such use of CD34+ stem cells in this indication. For example, CD34+ cells isolated from human PB after HGF mobilization stained positively for c-Troponin-T when transplanted directly into the scar of athymic rats with experimental MI, indicating they may also differentiate into cardiomyocytes [27]. In humans, endogenous CD34+ cells are released into the blood within the first hours following MI and for approximately one week, which seems to be a physiological response to limit ischemic scar formation [28].
We were the first to perform direct intra-cardiac delivery of large amounts of PB-CD34+ cells, previously purified by in vitro immune-selection, in patients suffering from bad-prognosis MI [29], and to further demonstrate the long-term benefit on regional heart structure and function of this breakthrough approach [24]. We also provided evidence that CD34bright cells could differentiate both endothelial and cardiac progenitor cells after in vitro culture on an appropriate and proprietary medium we had developed [24]. Other investigators followed the same direction, using CD34+ cells purified and enriched by immuno-selection either from PB after mobilization [30] or BM aspirates [31], but with less significant results, due to variations in the numbers of cells delivered, the route of injection, and the stage of the ischemic disease, which are key factors for successful therapy [32].
The cardiac repair mechanisms of CD34+ cells are likely multi-faceted. Once activated by a complex blend of cardio-active chemokines secreted by the inflammatory scar [33,34], injected CD34+ cells may release soluble paracrine factors and exosomes that can enhance the proliferation of resident cardiomyocytes [35] or neo-angiogenesis [36], respectively, thus reducing fibrosis and avoiding remodeling effects. The scar chemokines also chemoattract CD34+ stem cells to the ischemic zone and induce their commitment along the endothelial and cardiac pathways [33], which is strongly dependent on changes that occur in myocardial stiffness after AMI [34]. Such commitment cannot occur under steady-state conditions but is crucial for the induction of cardiac tissue repair after ischemic disease.
Considering these data, it now seems that some, if not all, CD34+ cells might be pluripotent rather than multipotent stem cells, capable of giving rise to a large panel of organic tissues under specific stimulations. This would be a more realistic explanation for their role in organ repair than the romantic concept of cellular plasticity, which may need to be rejected.
Very Small Embryonic-Like Stem Cells (VSELs)
For the first time in 2006, Ratajczak, et al. [37] described a type of cells which have kept their embryonic capacities in the bone marrow of adult mice [37]. Indeed, these cells, known as very small embryonic-like stem cells (VSELs) have similar characteristics to ESCs and are believed to originate from primordial germ cells in the yolk sac, after which they migrate, escape specification into tissue-committed stem cells, retain their pluripotency and settle in different organs and persist throughout life (reviewed in [38]). Their existence was further demonstrated in humans by the same group and several others [39-42]. Interestingly, VSELs are phenotypically characterized as being CD34+, CD133+ and/or CXCR4+, but do not express lineages (Lin–) and hematopoietic (CD45–) markers [37,43]. They can be sorted and isolated on the basis of their phenotypic features and their small size (5 to 6 μm). They express embryonic pluripotent stem cell-specific markers, such as SSEA-4 and TRA-1-81, on their surface, and Oct-4, Nanog and Sox-2 transcription factors at the protein level. VSELs constitute a rare and homogeneous pluripotent fraction of CD34+ stem cells and quiescent that prevents them from over-proliferation and potential risk of teratoma formation. Their quiescence under the steady state is related to the expression of low levels of genes involved in proliferation and cell signaling, which become upregulated during cell activation. In addition to the bone marrow and peripheral blood, VSELs have also been identified in umbilical cord blood and other organs [44], diversifying their source of collection and sampling. They can support vessel formation in vivo [45], and be specified to cardiomyocytes [46,47] neurons [48] and hematopoietic stem cells [49], both in vitro and in animal models.
Despite the huge reserves generated by this discovery, several laboratories were then able to characterize their biology and function when they were correctly isolated [41,42,50,51]. Populations similar or resembling VSELs have been described depending on the experimental strategies and diverse markers used for their isolation [52–54]. We observed that VSELs were positive for a pluripotent transcription factor, Nanog, isolated from umbilical cord blood. VSELs enclose heterogeneous cells expressing overlapping receptors; as such, a characterization of their biology is required [55]. We previously demonstrated that they circulate in very small numbers in peripheral blood under a steady state throughout life [56], but they can be mobilized in larger numbers by G-CSF from the BM into the PB [57]. Moreover, VSELs are actively mobilized from the bone marrow to the peripheral blood following stressful states such as myocardial infarction [58], in patients with leukemia [59] or stroke [60,61]. In Transwell chemotaxis assays, plasma collected from patients 6 h after AMI at hospital admission strongly chemo-attracted human bone marrow VSELs, suggesting that the AMI cardiac cells secrete specific chemoattractant growth factors [62].
Emerging evidence indicates that CD34+ VSELs could be at least in part responsible for the repairs and improvement of the cardiac function observed when using CD34+ cells in regenerative medicine after MI. Due to their properties, and as they are thought to be a reserve source of pluripotent CD34+ stem cells, their development could be used to ensure a safe, successful and efficient regenerative therapy for various non-curable diseases. Over the past several years, different reports indicated that in addition to HSCs, endothelial progenitor and mesenchymal stromal cells, VSELs are recruited to peripheral blood during MI, contributing to the repair of infarcted myocardium [63-65]. in vitro and in vivo studies have demonstrated through animal models that they are capable of regenerating several cell types, including cardiomyocytes and vascular endothelial cells, representing a serious alternative to other stem cell sources in cardiac regenerative trials. In acute experimental MI, in contrast to HSC CD45+, an intra-myocardial injection of VSELs is able to better attenuate left ventricular dysfunction and improve ejection fraction [46], as well as reduce myocardial hypertrophy [66]. A comparable effect was observed in chronic heart failure models [47]. Transplantation of VSELs-like MSCs triggered an improvement in cardiac function and heart remodeling in infarcted rats [67], similar to our data recorded with an expanded CD34+ cell graft [68]. Zuba-Surma, et al. [69] observed in mice a concomitant increase of pluripotent markers (Oct-4, Nanog, Rex-1 and Dppa1) and VSELs in PB 48 h after MI induction, whereas this was not observed for HSCs [69]. An increased expression of pluripotency transcripts specific to VSELs concomitant improvement of cardiac markers secreted on PB was observed after treatment by the natural anti-oxidant molecule resveratrol, suggesting that its beneficial effect on heart function is associated with the activation of VSELs. These data support further study and development of VSELs with a view to their use in cardiac regeneration. Finally, interest in VSELs as a source of stem cells for regenerative applications increased once some clinical studies demonstrated their therapeutic potential in patients with chronic heart failure [66,70].
Before considering the use of VSELs in regenerative medicine, it is necessary to resolve the problem of their limited number by finding the means to control their quiescence [71], thereby stimulating their capacity for proliferation without affecting their pluripotency. However, in order to determine and establish how to make them proliferate in the presence of an appropriate support without feeders, many groups looked for conditions suitable for their proliferation. A positive proliferative was observed in response to their stimulation by specific growth factors and gonadal sex hormones (nicotinamide, FSH, LH, BMP-4, FGF-2 and KL) [72,73], as well as with UM171, a pyrimidoindole derivative known to be able to induce the self-renewal of hematopoietic stem cells [74]. This had a positive effect on the expansion and proliferation of different populations of VSELs, significantly increasing their number without affecting their ability to differentiate into specific organ cells [55]. Given the phenotype of VSELs and their unique characteristics, purified CD34+ cells are presumed to contain small numbers of CD45- cells, with only a small fraction containing VSELs, which explains the need for a large numbers of cells to have a significant effect on cardiac repair.
Induced Pluripotent Stem Cells (iPSCs)
Among other challenging approaches to prevent or even reverse the destruction of cardiac tissue, induced pluripotent stem cells (iPSCs) technology has generated a great interest due to its potential feasibility in cardiac regenerative medicine. These cells were developed by Yamanaka’s research group in 2006 [75], based on the transduction of somatic mouse fibroblast with a cocktail of four genes (Oct4, Sox2, c-Myc and Klf4) that encode transcription factors governing pluripotency via integrating vectors (virus, lentivirus), resulting in the acquisition of the morphology and growth properties of ESCs. Yamanaka’s team and others have further demonstrated that human adult cells can also be reprogrammed to restore ESC characteristics by introducing the same or additional set of transcription factors [75,76]. Despite their ability to multiply indefinitely and to differentiate again in all cell types, they remain distinct from ESCs.
Avoiding any ethical issue, iPSCs constitute an exciting alternative source of pluripotent stem cells for disease modeling, pharmacological screening and cardiac cell therapy development. However, it has been demonstrated that low-passage of mouse iPSCs-derived by factor-based reprogramming harbors residual DNA methylation signatures, and maintains characteristics of their somatic tissue of origin as epigenetic memory (methylation) and genetic signature (that extends to miRNA), which may affect their lineage-specific differentiation capacity [77-79]. In addition, iPSCs generated with reprogramming factors via either integrating retroviruses or lentiviruses might cause insertional mutagenesis [80]. Furthermore, major issues regarding iPSCs have limited their clinical use, for example, their teratoma formation ability [81] resulting from their somatic cells of origin or occurring during the stepwise reprogramming process to iPSCs [82], and the risk of associated cancer formation linked to c-Myc and Klf4 known as potent oncogenes [83].
In order to increase the safety of iPSCs for clinical applications, human iPSCs technology has evolved through various non-integrative approaches, including reprogramming using episomal DNA [76,84], adenovirus [85], Sendai virus [86], PiggyBac transposon [87], a non-viral minicircle vector [88], use of recombinant proteins [89], or synthetically modified mRNAs [90] and micro RNAs [91]. All of these methods have made it possible to theoretically avoid the risk of insertional mutagenesis and genetic alterations [92].
Besides the hope generated by their use in the regeneration of the retina, iPSCs have aroused significant interest over the past decade in relation to cardiac repair, particularly in the treatment of myocardial infarction through regeneration. Ieda, et al. [93] were the first to describe a possible reprogramming of mouse fibroblasts into induced cardiomyocytes-like cells directly with the expression of three cardiogenic transcriptional factors (i.e., GATA-4, Mef2c and Tbx5), which may provide a source of cardiomyocytes for regenerative approaches [93]. However, only a small proportion of these converted cells display spontaneous contractions and express cardiomyocyte global gene expression profiles. Indeed, this technology was not sufficient for cardiac reprogramming in humans and needed other factors to be able to induce a cardiac-like phenotype [94,95].
The next challenge is to improve these findings and transfer the mouse direct reprogramming approach into humans, before their clinical application. To establish more efficient conditions for conversion of adult human fibroblasts to a cardiac phenotype, forced expression of cardiac transcription factors GATA-4, Hand2, TBX5, myocardin and Hand2 combined with muscle-specific microRNAs have shown that human fibroblasts can be reprogrammed to cardiac-like cells and displayed sarcomere-like structures and calcium transients; however, only a small subset of such cells exhibited spontaneous contractility [95]. Other strategies have been explored and revealed that an addition of Hand2 [96] and a constitutively active form of AKT to the identified cardiac transcription factors synergistically activated genome-wide cardiogenic stage-specific enhancers [97]. In addition, human fibroblasts could be converted into functional cardiomyocytes by a combination of nine small molecules, resulting in induced cardiomyocyte-like cells that uniformly contracted and resembled human cardiomyocytes in their transcriptome, epigenetic, and electrophysiological properties [98]. However, difficulties have been observed during the application of iPSCs in the heart field by the fact that all cells do not fully differentiate into cardiomyocytes and consequently are unable to restore massive loss of cardiac tissue responsible of fibrosis and scar formation [99].
Recent studies have shown that combining three human iPSCs-derived cardiac cell types in a three-dimensional micro-tissues could improve sarcomeric structures with T-tubules, enhance contractility and mitochondrial respiration, and promote electrical maturation associated to connexin 43 (CX43 gap junction) and intracellular cyclic AMP (cAMP) pathway activation [100]. It has been also demonstrated that sustained activation of AMP-activated protein kinase in human iPSC-derived cardiomyocytes via Sirtuin activation improved differentiation, leading to decreased acetylation of histones H3 (at Lys9 and 56) and H4 (at Lys16), and increased mRNA and protein expression of both TNNI3 and TNNT2 [101]. Given the problems with rejection that can arise during the use of iPSCs due to the need for MHC matching and immunosuppressive treatment [102], another debate has emerged regarding the use of autologous versus allogeneic iPSCs. The autologous setting implies a selection of cells specific to the patient, making their use lengthy, expensive, laborious and regulated. Indeed, generating single graft iPSCs from proper patient cells could take several months of preparation, which may be unrealistic for the treatment of diseases such as MI. In contrast, transplanted allogenic iPSCs-derived cardiomyocytes, in association with immunosuppressors, can survive for up to 12 weeks in a macaque heart, but these animals presented ventricular tachycardia, which could be due to the immature state of the transplanted cells [103].
Although some reports have demonstrated that cardiomyocytes derived from iPSCs can be grafted into myocardium of animals and improve left ventricular function [104,105], in most cases iPSCs differentiate into immature cardiomyocytes with potential arrhythmic complications rather than to adult functional cardiomyocytes harboring their structures and gene expression profiles, particularly in larger animals [103,106,107]. A pilot study using allogeneic iPSCs-derived cardiomyocytes directly injected in the myocardium during a CABG operation has been recently launched in China [108], but is still in the recruitment process of five patients with ischemic heart disease. In addition, other groups have reported that iPSCs do not directly contribute to the regeneration of cardiac tissues but are sources of growth factors that provide only a paracrine effect [109-111]. Recent studies using a mouse model of MI show that the transplantation of iPSCs can improve heart function via paracrine action [112]. In addition, iPSC-derived extracellular vesicles induce superior cardiac repair in vivo than cell transplantation, representing a safer alternative [113]. In 2018, the Japanese government approved a world-first study in which the investigators prefer to surgically implant degradable sheets of heart muscle tissues made from allogeneic iPSCs onto the external surface of the infarcted area as a source of growth factors, micro-vesicles, or exosomes, rather than integrating iPSCs-derived cardiomyocytes into the host cardiac tissue [114]. The first of the ten patients scheduled was treated at the beginning of 2020, but no data are available yet.
Conclusion
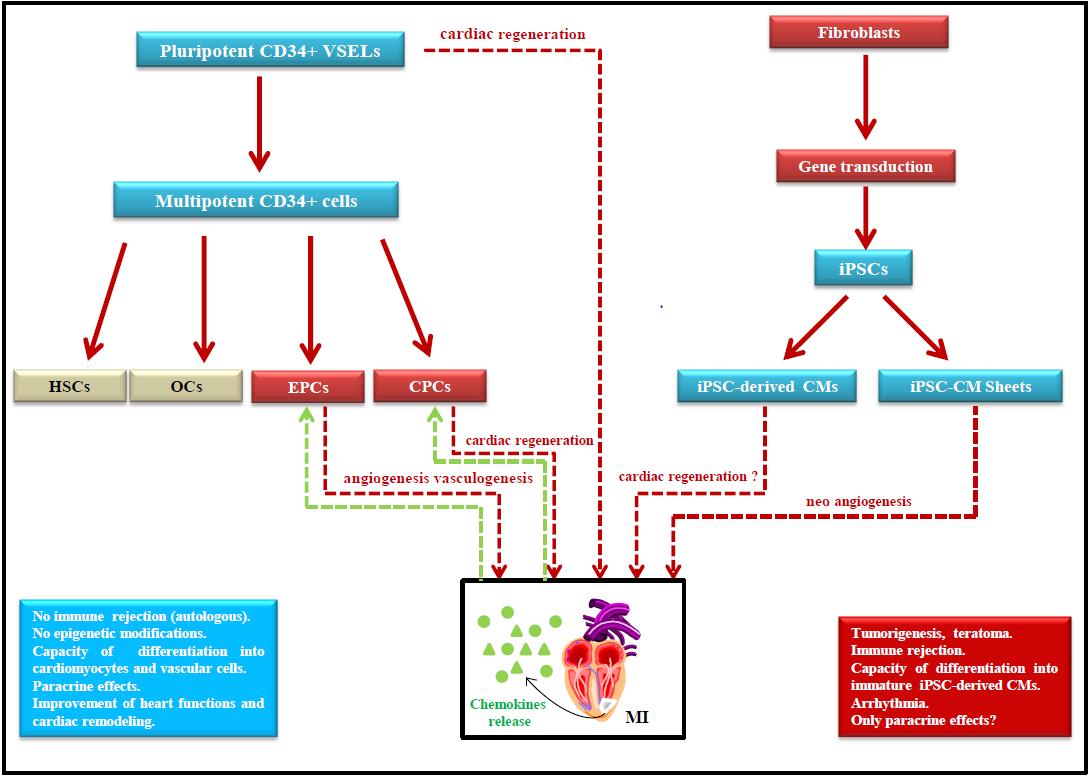
Besides ESCs, other cell types have been retained by different groups of researchers (i.e., CD34+, VSELs and iPSCs) to determine the benefits and drawbacks related to their future development with a view to applications in cardiac regeneration (Figure 1). Purified, CD34+ stem cells taken from blood or bone marrow have displayed effectiveness in the treatment of blood diseases, but other unexpected therapeutic dimensions related to these cells have been identified in recent years. Despite the emergence of iPSCs and the hope they have aroused as an alternative to overcome the frustrating problems raised by ESCs, no clinical trial has been successfully achieved to date. The main hurdles to overcome are the risk of tumorigenesis, the development of life-threatening arrhythmias and immunogenicity. Moreover, engineering iPSCs is a lengthy and costly process. The choice of manufacturing surgically implantable cardiac sheets from iPSCs, with the only goal being to achieve a hypothetic paracrine effect promoting neo-angiogenesis, rather than cardiac tissue repair, is likely clinically and economically not very acceptable. The iPSCs should not be realistically employed in the clinic in the foreseeable future until significant progress in their clinical safety has been achieved. At the moment, they only serve as models to test in vitro drugs’ toxic effects on cell survival, genetics, or differentiation, or as tools for developing experimental models to study genetic diseases. Even in such cases, data obtained using iPSCs must be cautiously evaluated because of their genomic instability and variability.
It is worth considering whether isolated and expanded VSELs could be safer and more efficient to improve heart function and cardiac remodeling when used in regenerative medicine. They represent a credible alternative to ESCs and iPSCs, as an easier-to-access source of purified pluripotent CD34+ stem cells for regenerative medicine, with no ethical issues, no epigenetic modifications and no undesirable side effects as risk of teratoma or cancer formation. Furthermore, CD34+ cells—consisting of about one fifth of VSELs—have already been demonstrated to be effective as a treatment for MI [24]. However, technical issues remain for VSELs, particularly the necessity to improve expansion-folds to achieve an optimal number of cells. Additional studies are therefore needed to prove the clinical potential of purified VSELs.
- GR Martin (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78: 7634-8.
- JA Thomson, J Itskovitz-Eldor, SS Shapiro, MA Waknitz, JJ Swiergiel, et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282: 1145-7.
- JE Visvader, H Clevers (2016) Tissue-specific designs of stem cell hierarchies. Nat Cell Biol 18: 349-55.
- R Dresser (2010) Stem cell research as innovation: expanding the ethical and policy conversation. J Law Med Ethics 38: 332-41.
- HL Thompson, JO Manilay (2011) Embryonic stem cell-derived hematopoietic stem cells: challenges in development, differentiation, and immunogenicity. Curr Top Med Chem 11: 1621-37.
- Z Storchova (2016) Too much to differentiate: aneuploidy promotes proliferation and teratoma formation in embryonic stem cells. EMBO J 35: 2265-7.
- H Stachelscheid, A Wulf-Goldenberg, K Eckert, J Jensen, J Edsbagge, et al. (2013) Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors, J Tissue Eng Regen Med 7: 729-41.
- K English, KJ Wood (2011) Immunogenicity of embryonic stem cell-derived progenitors after transplantation, Curr Opin Organ Transplant 16: 90-5.
- P Menasché, V Vanneaux, A Hagège, A Bel, B Cholley, et al. (2018) Transplantation of Human Embryonic Stem Cell–Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction, Journal of the American College of Cardiology 71: 429-38.
- D Orlic, J Kajstura, S Chimenti, I Jakoniuk, SM Anderson, et al. (2001) Bone marrow cells regenerate infarcted myocardium. Nature 410: 701-5.
- EC Perin, JT Willerson, CJ Pepine, TD Henry, SG Ellis, et al. (2012) Cardiovascular Cell Therapy Research Network (CCTRN), Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA 307: 1717-26.
- M Al-Nbaheen, R Vishnubalaji, D Ali, A Bouslimi, F Al-Jassir, et al. (2013) Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev Rep 9: 32-43.
- MS Khubutiya, AV Vagabov, AA Temnov, AN Sklifas (2014) Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy 16: 579-85.
- JH van Berlo, O Kanisicak, M Maillet, RJ Vagnozzi, J Karch, et al. (2014) c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509: 337-41.
- CI Civin, LC Strauss, C Brovall, MJ Fackler, JF Schwartz, et al. (1984) Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol 133: 157-65.
- DS Krause, MJ Fackler, CI Civin, WS May (1996) CD34: structure, biology, and clinical utility. Blood 87: 1-13.
- CI Civin, G Almeida-Porada, MJ Lee, J Olweus, LW Terstappen, et al. (1996) Sustained, retransplantable, multilineage engraftment of highly purified adult human bone marrow stem cells in vivo. Blood 88: 4102-9.
- G Herbein, H Sovalat, E Wunder, M Baerenzung, J Bachorz, et al. (1994) Isolation and identification of two CD34+ cell subpopulations from normal human peripheral blood. Stem Cells 12: 187-97.
- LB To, PG Dyson, CA Juttner (1986) Cell-dose effect in circulating stem-cell autografting. Lancet 2: 404-5.
- A Debecker, P Henon, M Lepers, JC Eisenmann, J Selva (1986) Collection of circulating stem cells during remission after chemotherapy in acute leukemia. Nouv Rev Fr Hematol 28: 287-92.
- PR Henon, A Butturini, RP Gale (1991) Blood-derived haematopoietic cell transplants: blood to blood?. Lancet 337: 961-3.
- P Hénon, H Sovalat, M Becker, Y Arkam, M Ojeda-Uribe, et al. (1998) Primordial role of CD34+ 38- cells in early and late trilineage haemopoietic engraftment after autologous blood cell transplantation. Br J Haematol 103: 568-81.
- T Asahara, T Murohara, A Sullivan, M Silver, R van der Zee, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964-7.
- S Pasquet, H Sovalat, P Hénon, N Bischoff, Y Arkam, et al. (2009) Long-term benefit of intracardiac delivery of autologous granulocyte–colony-stimulating factor-mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy 11: 1002-15.
- MY Gordon, N Levicar, M Pai, P Bachellier, I Dimarakis, et al. (2006) Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells 24: 1822-30.
- T Matsumoto, R Kuroda, Y Mifune, A Kawamoto, T Shoji, et al. (2008) Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone 43: 434-9.
- H Iwasaki, A Kawamoto, M Ishikawa, A Oyamada, S Nakamori, et al. (2006) Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 113: 1311-25.
- HD Theiss, R David, MG Engelmann, A Barth, K Schotten, et al. (2007) Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM). Eur Heart J 28: 1258-64.
- P Hénon, M Ojeda-Uribe, Y Arkam, H Sovalat, N Bischoff, et al. (2003) Intra-cardiac reinjection of purified autologous blood CD34+ cells mobilized by G-CSF can significantly improve myocardial function in cardiac patients. Blood 102: 335a.
- DW Losordo, TD Henry, C Davidson, JS Lee, MA Costa, et al. (2011) Intramyocardial, Autologous CD34+ Cell Therapy for Refractory Angina. Circulation Res 109: 428–36.
- AA Quyyumi, EK Waller, J Murrow, F Esteves, J Galt, et al. (2011) CD34+ cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J 161: 98-105.
- P Hénon (2020) Key Success Factors for Regenerative Medicine in Acquired Heart Diseases. Stem Cell Rev Rep 16: 441-58.
- H Ebelt, M Jungblut, Y Zhang, T Kubin, S Kostin, et al. (2007) Cellular cardiomyoplasty: improvement of left ventricular function correlates with the release of cardioactive cytokines. Stem Cells 25: 236-44.
- HJ Cho, N Lee, JY Lee, YJ Choi, M Ii, et al. (2007) Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med 204: 3257-69.
- MZ Ratajczak, J Ratajczak, M Kucia (2019) Very Small Embryonic-Like Stem Cells (VSELs): An Update and Future Directions. Circulation Research 124: 208-10.
- S Sahoo, E Klychko, T Thorne, S Misener, KM Schultz, et al. (2011) Exosomes From Human CD34 + Stem Cells Mediate Their Proangiogenic Paracrine Activity. Circulation Research 109: 724-8.
- M Kucia, R Reca, FR Campbell, E Zuba-Surma, M Majka, et al. (2006) A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 20: 857-69.
- MZ Ratajczak (2017) Why are hematopoietic stem cells so ‘sexy’? on a search for developmental explanation. Leukemia 31: 1671-7.
- SH Kassmer, H Jin, PX Zhang, EM Bruscia, K Heydari, et al. (2013) Very small embryonic-like stem cells from the murine bone marrow differentiate into epithelial cells of the lung. Stem Cells 31: 2759-66.
- AM Havens, H Sun, Y Shiozawa, Y Jung, J Wang, et al. (2014) Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo. Stem Cells Dev 23: 689-701.
- CL Guerin, X Loyer, J Vilar, A Cras, T Mirault, et al. (2015) Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: evidence of vasculogenic potential. Thrombosis and Haemostasis 113: 1084-94.
- A Shaikh, P Nagvenkar, P Pethe, I Hinduja, D Bhartiya (2015) Molecular and phenotypic characterization of CD133 and SSEA4 enriched very small embryonic-like stem cells in human cord blood. Leukemia 29: 1909-17.
- MZ Ratajczak, DM Shin, R Liu, K Mierzejewska, J Ratajczak, et al. (2012) Very small embryonic/epiblast-like stem cells (VSELs) and their potential role in aging and organ rejuvenation – an update and comparison to other primitive small stem cells isolated from adult tissues. Aging 4: 235-46.
- M Kucia, M Halasa, M Wysoczynski, M Baskiewicz-Masiuk, S Moldenhawer, et al. (2007) Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia 21: 297-303.
- CL Guerin, E Rossi, B Saubamea, A Cras, V Mignon, et al. (2017) Human very Small Embryonic-like Cells Support Vascular Maturation and Therapeutic Revascularization Induced by Endothelial Progenitor Cells. Stem Cell Reviews and Reports 13: 552-60.
- B Dawn, S Tiwari, MJ Kucia, EK Zuba-Surma, Y Guo, et al. (2008) Transplantation of Bone Marrow-Derived Very Small Embryonic-Like Stem Cells Attenuates Left Ventricular Dysfunction and Remodeling After Myocardial Infarction. Stem Cells 26: 1646-55.
- EK Zuba‐Surma, Y Guo, H Taher, SK Sanganalmath, G Hunt, et al. (2011) Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cellular Molecul Med 15: 1319-28.
- SJ Lee, SH Park, YI Kim, S Hwang, PM Kwon, et al. (2014) Adult Stem Cells from the Hyaluronic Acid-Rich Node and Duct System Differentiate into Neuronal Cells and Repair Brain Injury. Stem Cells and Development 23: 2831-40.
- J Ratajczak, M Wysoczynski, E Zuba-Surma, W Wan, M Kucia, et al. (2011) Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Experimental Hematology 39: 225-37.
- SH Kassmer, DS Krause (2013) Very small embryonic-like cells: Biology and function of these potential endogenous pluripotent stem cells in adult tissues: Very Small Embryonic-like Stem Cells. Molecular Reproduction and Development 80: 677-90.
- R Nakatsuka, R Iwaki, Y Matsuoka, K Sumide, H Kawamura, et al. (2016) Identification and Characterization of Lineage − CD45 − Sca-1 + VSEL Phenotypic Cells Residing in Adult Mouse Bone Tissue. Stem Cells and Development 25: 27-42.
- G D’Ippolito, S Diabira, GA Howard, P Menei, BA Roos, et al. (2004) Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 117: 2971-81.
- G Kögler, S Sensken, JA Airey, T Trapp, M Müschen, et al. (2004) A New Human Somatic Stem Cell from Placental Cord Blood with Intrinsic Pluripotent Differentiation Potential. J Exp Med 200: 123-35.
- AP Beltrami, D Cesselli, N Bergamin, P Marcon, S Rigo, et al. (2007) Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood 110: 3438-46.
- R Lahlil, M Scrofani, R Barbet, C Tancredi, A Aries, et al. (2018) VSELs Maintain their Pluripotency and Competence to Differentiate after Enhanced Ex Vivo Expansion. Stem Cell Rev Rep 14: 510-24.
- H Sovalat, M Scrofani, A Eidenschenk, P Hénon (2016) Human Very Small Embryonic-Like Stem Cells Are Present in Normal Peripheral Blood of Young, Middle-Aged, and Aged Subjects. Stem Cells Int 2016: 1-8.
- H Sovalat, M Scrofani, A Eidenschenk, S Pasquet, V Rimelen, et al. (2011) Identification and isolation from either adult human bone marrow or G-CSF−mobilized peripheral blood of CD34+/CD133+/CXCR4+/ Lin−CD45− cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Experimental Hematology 39: 495-505.
- A Abdel-Latif, EK Zuba-Surma, KM Ziada, M Kucia, DA Cohen, et al. (2010) Evidence of mobilization of pluripotent stem cells into peripheral blood of patients with myocardial ischemia. Experimental Hematology 38: 1131-42.
- A Eljaszewicz, L Bolkun, K Grubczak, M Rusak, T Wasiluk, et al. (2018) Very Small Embryonic-Like Stem Cells, Endothelial Progenitor Cells, and Different Monocyte Subsets Are Effectively Mobilized in Acute Lymphoblastic Leukemia Patients after G-CSF Treatment. Stem Cells Int 2018: 1943980.
- CV Borlongan, LE Glover, N Tajiri, Y Kaneko, TB Freeman (2011) The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol 95: 213-28.
- K Grymula, M Tarnowski, K Piotrowska, M Suszynska, K Mierzejewskaet al. (2014) Evidence that the population of quiescent bone marrow-residing very small embryonic/epiblast-like stem cells (VSELs) expands in response to neurotoxic treatment. J Cell Mol Med 18: 1797-806.
- AV Karapetyan, YM Klyachkin, S Selim, M Sunkara, KM Ziada, et al. (2013) Bioactive lipids and cationic antimicrobial peptides as new potential regulators for trafficking of bone marrow-derived stem cells in patients with acute myocardial infarction. Stem Cells Dev 22: 1645-56.
- M Kucia, B Dawn, G Hunt, Y Guo, M Wysoczynski, et al. (2004) Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res 95: 1191-9.
- W Wojakowski, M Tendera, A Michałowska, M Majka, M Kucia, et al. (2004) Mobilization of CD34/CXCR4 + , CD34/CD117 + , c-met + Stem Cells, and Mononuclear Cells Expressing Early Cardiac, Muscle, and Endothelial Markers Into Peripheral Blood in Patients With Acute Myocardial Infarction. Circulation 110: 3213-20.
- N Kränkel, RG Katare, M Siragusa, LS Barcelos, P Campagnolo, et al. (2008) Role of Kinin B 2 Receptor Signaling in the Recruitment of Circulating Progenitor Cells With Neovascularization Potential. Circulation Research 103: 1335-43.
- W Wojakowski, M Kucia, E Zuba-Surma, T Jadczyk, B Książek, et al. (2011) Very small embryonic-like stem cells in cardiovascular repair. Pharmacol Therap 129: 21-8.
- S Zhang, L Zhao, J Wang, N Chen, J Yan, et al. (2017) HIF-2α and Oct4 have synergistic effects on survival and myocardial repair of very small embryonic-like mesenchymal stem cells in infarcted hearts. Cell Death Dis 8: e2548.
- C Saucourt, S Vogt, A Merlin, C Valat, A Criquet, et al. (2019) Design and Validation of an Automated Process for the Expansion of Peripheral Blood‐Derived CD34 + Cells for Clinical Use After Myocardial Infarction. STEM CELLS Trans Med 8: 822-32.
- EK Zuba-Surma, M Kucia, B Dawn, Y Guo, MZ Ratajczak, et al. (2008) Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cellular Cardiol 44: 865-73.
- M Tendera, W Wojakowski, W Ruz, C Ke, J Nessler, et al. (2009) Intracoronary infusion of bone marrow-derived selected CD341CXCR41 cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Europ Heart J 30: 1313-21.
- C Alvarez-Gonzalez, R Duggleby, B Vagaska, S Querol, SG Gomez, et al. (2013) Cord Blood Lin−CD45− Embryonic-Like Stem Cells Are a Heterogeneous Population That Lack Self-Renewal Capacity. PLoS ONE 8: e67968.
- MZ Ratajczak, J Ratajczak, M Suszynska, DM Miller, M Kucia, et al. (2017) A Novel View of the Adult Stem Cell Compartment From the Perspective of a Quiescent Population of Very Small Embryonic-Like Stem Cells. Circul Res 120: 166-78.
- MZ Ratajczak, A Bartke, Z Darzynkiewicz (2017) Prolonged Growth Hormone/Insulin/Insulin-like Growth Factor Nutrient Response Signaling Pathway as a Silent Killer of Stem Cells and a Culprit in Aging, Stem Cell Rev Rep 13: 443-53.
- I Fares, J Chagraoui, Y Gareau, S Gingras, R Ruel, et al. (2014) Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal, Science 345: 1509-12.
- K Takahashi, K Tanabe, M Ohnuki, M Narita, T Ichisaka, et al. (2007) Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 131: 861-72.
- J Yu, MA Vodyanik, K Smuga-Otto, J Antosiewicz-Bourget, JL Frane, et al. (2007) Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 318: 1917-20.
- MH Chin, MJ Mason, W Xie, S Volinia, M Singer, et al. (2009) Induced Pluripotent Stem Cells and Embryonic Stem Cells Are Distinguished by Gene Expression Signatures. Cell Stem Cell 5: 111-23.
- JM Polo, S Liu, ME Figueroa, W Kulalert, S Eminli, et al. (2010) Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 28: 848-55.
- EY Kim, K Jeon, HY Park, YJ Han, BC Yang, et al. (2010) Differences between cellular and molecular profiles of induced pluripotent stem cells generated from mouse embryonic fibroblasts. Cell Reprogram 12: 627-39.
- S Yamanaka (2012) Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell 10: 678-84.
- S Masuda (2012) Risk of Teratoma Formation After Transplantation of Induced Pluripotent Stem Cells. Chest 141:1120-21.
- U Ben-David, N Benvenisty (2011) The tumorigenicity of human embryonic and induced pluripotent stem cells, Nature Rev Can 11: 268-77.
- S Attwood, M Edel (2019) iPS-Cell Technology and the Problem of Genetic Instability—Can It Ever Be Safe for Clinical Use?. J Clin Med 8: 288.
- K Okita, Y Matsumura, Y Sato, A Okada, A Morizane, et al. (2011) A more efficient method to generate integration-free human iPS cells, Nat. Methods 8: 409-12.
- M Stadtfeld, M Nagaya, J Utikal, G Weir, K Hochedlinge (2008) Induced Pluripotent Stem Cells Generated Without Viral Integration. Science 322: 945-9.
- N Fusaki, H Ban, A Nishiyama, K Saeki, M Hasegawa (2009) Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B 85: 348-62.
- K Woltjen, IP Michael, P Mohseni, R Desai, M Mileikovsky, et al. (2009) piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458: 766-70.
- F Jia, KD Wilson, N Sun, DM Gupta, M Huang, et al. (2010) A nonviral minicircle vector for deriving human iPS cells. Nature Methods 7: 197-9.
- D Kim, CH Kim, JI Moon, YG Chung, MY Chang, et al. (2009) Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell 4: 472-6.
- L Warren, PD Manos, T Ahfeldt, YH Loh, H Li, et al. (2010) Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell 7: 618-30.
- N Miyoshi, H Ishii, H Nagano, N Haraguchi, DL Dewi, et al. (2011) Reprogramming of Mouse and Human Cells to Pluripotency Using Mature MicroRNAs. Cell Stem Cell 8: 633-8.
- Y Shi, H Inoue, JC Wu, S Yamanaka (2017) Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16: 115-30.
- M Ieda, JD Fu, P Delgado-Olguin, V Vedantham, Y Hayashi, et al. (2010) Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors. Cell 142: 375-86.
- R Wada, N Muraoka, K Inagawa, H Yamakawa, K Miyamoto, et al. (2013) Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proceedings of the National Academy of Sciences 110: 12667-72.
- YJ Nam, K Song, X Luo, E Daniel, K Lambeth, et al. (2013) Reprogramming of human fibroblasts toward a cardiac fate. Proceedings of the National Academy of Sciences 110: 5588-93.
- K Song, YJ Nam, X Luo, X Qi, W Tan, et al. (2012) Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485: 599-604.
- H Hashimoto, Z Wang, GA Garry, VS Malladi, GA Botten, et al. (2019) Cardiac Reprogramming Factors Synergistically Activate Genome-wide Cardiogenic Stage-Specific Enhancers. Cell Stem Cell 25: 69-86.e5.
- N Cao, Y Huang, J Zheng, CI Spencer, Y Zhang, et al. (2016) Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science 352: 1216-20.
- X Yang, L Pabon, CE Murry (2014) Engineering Adolescence: Maturation of Human Pluripotent Stem Cell–Derived Cardiomyocytes. Circulation Res 114: 511-23.
- E Giacomelli, V Meraviglia, G Campostrini, A Cochrane, X Cao, et al. (2020) Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell 26: 862-79.e11.
- M Sarikhani, JC Garbern, S Ma, R Sereda, J Conde, et al. (2020) Sustained Activation of AMPK Enhances Differentiation of Human iPSC-Derived Cardiomyocytes via Sirtuin Activation. Stem Cell Rep 15: 498-514.
- T Kawamura, S Miyagawa, S Fukushima, A Maeda, N Kashiyama, et al. (2016) Cardiomyocytes Derived from MHC-Homozygous Induced Pluripotent Stem Cells Exhibit Reduced Allogeneic Immunogenicity in MHC-Matched Non-human Primates. Stem Cell Rep 6: 312-20.
- Y Shiba, T Gomibuchi, T Seto, Y Wada, H Ichimura, et al. (2016) Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538: 388-91.
- M Kawamura, S Miyagawa, K Miki, A Saito, S Fukushima, et al. (2012) Feasibility, Safety, and Therapeutic Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Sheets in a Porcine Ischemic Cardiomyopathy Model. Circulation 126: S29-37.
- Y Jiang, XL Lian (2020) Heart regeneration with human pluripotent stem cells: Prospects and challenges. Bioactive Materials 5: 74-81.
- JJH Chong, X Yang, CW Don, E Minami, YW Liu, et al. (2014) Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510: 273-7.
- JJH Chong, CE Murry (2014) Cardiac regeneration using pluripotent stem cells—Progression to large animal models. Stem Cell Res 13: 654-65.
- S Kobold, A Guhr, N Mah, N Bultjer, S Seltmann, et al. (2020) A Manually Curated Database on Clinical Studies Involving Cell Products Derived from Human Pluripotent Stem Cells. Stem Cell Rep 15: 546-55.
- A Tachibana, MR Santoso, M Mahmoudi, P Shukla, L Wang, et al. (2017) Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circulation Res 121.
- PJ Kim, M Mahmoudi, X Ge, Y Matsuura, I Toma, et al. (2015) Direct Evaluation of Myocardial Viability and Stem Cell Engraftment Demonstrates Salvage of the Injured Myocardium. Circulation Res 116.
- SG Ong, BC Huber, W Hee Lee, K Kodo, AD Ebert, et al. (2015) Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation 132: 762-71.
- X Jiang, Z Yang, M Dong (2020) Cardiac repair in a murine model of myocardial infarction with human induced pluripotent stem cell-derived cardiomyocytes, Stem Cell Res Ther 11.
- M Adamiak, G Cheng, S Bobis-Wozowicz, L Zhao, S Kedracka-Krok, et al. (2018) Induced Pluripotent Stem Cell (iPSC)–Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circulation Res 122: 296-309.
- D Cyranoski (2018) ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 557: 619-20.

Figures at a glance