Cystathionine-β-Synthase Deficiency Revealed by Recurrent Thrombosis in Young Patients: A Diagnosis often Missed
Received Date: July 03, 2020Accepted Date: August 01, 2020 Published Date: August 03, 2020
doi: 10.17303/jcvm.2020.6.301
Citation:Maynadie Hortense (2020) Cystathionine-β-Synthase Deficiency Revealed by Recurrent Thrombosis in Young Patients: A Diagnosis often Missed. J Cardio Vasc Med 6: 1-8.
Abstract
Homocystinuria is a rare inherited disorder characterized by cystathionine beta-synthase deficiency. Undiagnosed and untreated patients have serious symptoms, such as arterial and venous thrombosis, osteoporosis, convulsions, and eye problems. At a young age, clinical signs can be mild and remain unnoticed by physicians, thus significantly delaying diagnosis and treatment. Vascular complications are the main cause of morbidity and mortality. The molecular mechanisms of thrombosis in these patients are not fully understood, but blood clotting changes are frequently observed: endothelial damage, platelet activation, and short- or long-term consumption of clotting factors and inhibitors, such as antithrombin and protein C. Here, we report four clinical cases in whom the diagnosis of homocystinuria was made after several episodes of thrombosis. This stresses the importance to make hematologists and vascular medicine specialists aware of the symptoms of this rare and severe thrombotic disorder in order to improve the management of a disease that can be easily and successfully treated after correct diagnosis.
Keywords: Homocystinuria; cystathionine beta-synthase; thrombophilia; antithrombin; venous thromboembolism
Key Message: The diagnosis of homocystinuria is often delayed and made after several episodes of recurrent thrombosis. The knowledge of its syndromic character and the associated clinical signs may lead to earlier diagnosis and better management of this rare inherited disorder by hematologists and vascular medicine specialists.
List of abbreviations: AT: antithrombin activity; CBS: cystathionine-β-synthase; CVT: cerebral venous thrombosis; DOAC: direct oral anticoagulant; DVT: Deep Vein Thrombosis; FVII: factor VII; INR: International normalized ratio; MRI: magnetic resonance imaging; MTHFR: methylene tetrahydrofolate reductase; PC: protein C; PE: pulmonary embolism; PS: protein S; VKA: Vitamin K Antagonists; VTE: Venous thromboembolism
Introduction
Homocystinuria due to cystathionine β-synthase (CBS) deficiency is a disorder of methionine metabolism characterized by severe hyperhomocysteinemia. It is a rare recessive inherited disorder with a prevalence from 1:1800 to 1:900000 in the function of the ethnicity and the method of diagnosis (e.g. genetic or biochemical screening in newborns or diagnosis of symptomatic patients) [1]. Due to the wide range of clinical presentations and symptom severity, its diagnosis is often delayed. The most common clinical form of the disease is characterized by ectopia lentis, myopia, osteoporosis, and developmental delay. Some patients may present intellectual disability, Marfanoid features, seizures, megaloblastic anemia, and skeletal abnormalities. Thromboembolic events are the leading cause of morbidity and mortality in patients with homocystinuria [1,2]. Venous thromboembolism (VTE) is the most frequent vascular complication, but thromboembolic events may occur also in arteries, and affect large and small vessels. Serious complications of thromboembolism have been reported, including hypertension due to renal infarcts, focal neurological signs secondary to cerebrovascular thrombi, myocardial infarction, and fatal pulmonary embolism [2].
The diagnosis of CBS deficiency is based on the detection of very high plasma levels of total homocysteine (>100µmol/L – normal values in adults < 15 µmol/L)) [3] associated with increased levels of methionine and decreased levels of cysteine. The diagnosis should be confirmed by CBS gene mutation analysis or by measuring CBS activity in plasma or in cultured fibroblasts. Hyperhomocystein emiacan also be caused by vitamin B12 and B9 deficiencies, renal failure, or inherited homocysteine re-methylation disorders, such as in individuals carrying the C677T mutation in the methylene tetrahydrofolate reductase (MTHFR) gene. However, in these cases, homocysteinemia is usually mild or moderate (between 15 and100 µmol/L), is not associated with homocystinuria, and is considered more a risk factor for cardiovascular diseases [3].
The treatment of CBS deficiency mainly relies on the supplementation of pyridoxine (CBS cofactor), folic acid, and cobalamin. However, 50% of patients do not respond to pyridoxine and require betaine and a methionine-restricted diet [1].
The presence of typical clinical signs and the involvement of four organ systems (eye, central nervous system, skeletal, and vascular systems) can easily lead to the suspicion of CBS deficiency. However, many patients with homocystinuria may only present recurrent VTE from a young age as the major clinical sign, with or without associated symptoms, such as myopia, osteoporosis, or neurological symptoms that may go unnoticed by physicians. This makes it difficult and delays the diagnosis of homocystinuria. Here, we describe four cases that emphasize the syndromic character of homocystinuria in order to make physicians aware of this rare condition and facilitate its diagnosis.
Case 1
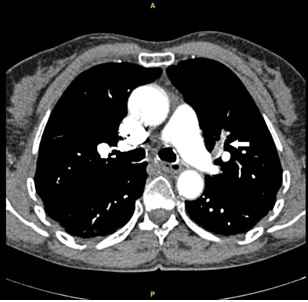
The first patient was a 52-year-old woman who had seven spontaneous VTE episodes (n=3 pulmonary embolism (PE), n=3 Deep Vein Thrombosis (DVT), and n= 1 superficial vein thrombosis). When she was 17-year-old, she presented a spontaneous DVT complicated by massive PE with pulmonary infarction. After the second spontaneous DVT, long-term anticoagulation therapy with Vitamin K Antagonists (VKA) was initiated. The five other VTE episodes occurred spontaneously, one during bridge therapy for a breast biopsy, and another episode while the International Normalized Ratio (INR) was below the target range of 2-3. She had no thrombosis risk factors, such as obesity, oral contraceptive use, pregnancy, or cancer. Routine thrombophilia testing reported no inherited or acquired thrombophilia, particularly, no antiphospholipid syndrome, no factor V Leiden mutation, no prothrombin gene mutation, no protein C (PC) or protein S (PS) deficiency. Antithrombin activity (AT) was at the lower limit of the reference range (Table 1). No thrombocytosis or polycythemia was detected, but macrocytosis was noticed. The diagnosis of homocystinuria was established after the last VTE episode, a bilateral segmental PE (Figure 1) that occurred despite stable INR within the target range of 2-3 and in the absence of any identified transient risk factor. Laboratory testing to detect rare thrombophilic defects did not find the JAK2 V617F mutation. Conversely, the total homocysteine levels in plasma were 464 µmol/L. After the diagnosis of homocystinuria, supplementation with cobalamin and folic acid was initiated. After 16 months of treatment, plasma homocysteine levels remained very high (299µmol/L). Therefore, the plasma amino acid profile was analyzed by chromatography that showed the specific profile of CBS deficiency with very high plasma homocysteine levels associated with increased methionine and reduced cysteine (Table 1) Pyridoxine and cysteine supplementation was initiated that rapidly normalized plasma homocysteine level (11 µmol/L) after 3 weeks of treatment. Interestingly, plasma AT also was normalized (111%) concomitantly with homocysteine levels.
A review of her medical records after diagnosis suggested that her history of seizures, cognitive deficiency, and depression could be related to homocystinuria. Moreover, a retinal detachment linked to a dislocated lens was also found in her medical records.
Case 2
A 33-year-old man was diagnosed with homocystinuria after two spontaneous and one surgery-induced DVT, on long-term therapy with VKA. The patient was a smoker and had IgA nephropathy. Routine thrombophilia testing detected only AT deficiency (Table 1). The level of total homocysteine was above 700µmol/L, and amino acid chromatography suggested CBS deficiency (very high levels of homocysteine, increased methionine, and reduced cysteine). Genetic testing revealed two heterozygous mutations in the CBS gene: p.Ile278Thr that is one of the most prevalent mutations responsible for pyridoxine-sensitive homocystinuria, and p.Leu230Arg that induces a premature stop codon [4]. He was treated with pyridoxine 1500mg daily, cobalamin 1mg daily, and folic acid 10mg daily. In this patient, the variations in homocysteine levels were caused by severe kidney disease. After kidney transplantation, the homocysteine level remained at 80 µmol/L. It was decided to stop the long-term anticoagulation therapy, but unfortunately, the patient had a new DVT of the calf veins complicated by PE. Long-term anticoagulation with VKA was reintroduced. In parallel, a treatment with betaine supplementation and methionine-restricted diet was initiated. The treatment led to a rapid decrease in plasma homocysteine level to 58 µmol/L.
After homocystinuria diagnosis, bone deformities in the left leg and foot were detected, without any other typical homocystinuria symptom.
Case 3
Homocystinuria was diagnosed in a 40-year-old woman after two VTE episodes induced by transient risk factors: a femoral and iliac DVT at the age of 21 years after starting combined oral contraceptive therapy, and a postoperative femoral vein DVT after cesarean section. The patient was a smoker and consumed alcohol regularly. After the second VTE episode, long-term anticoagulation therapy (VKA) was initiated. Thrombophilia testing showed a slight and persistent decrease in AT activity (76%), but no other thrombophilic disorder (Table 1). Blood count highlighted the presence of isolated macrocytosis without any other abnormality. The total homocysteine level was above 200 µmol/L. CBS deficiency was diagnosed using fibroblasts isolated from a skin biopsy. Genetic testing identified compound heterozygosity for the p.Ile278Thr and p.Leu230Arg CBS mutations.The diagnosis was confirmed 17 years after the first VTE, and specific treatment was initiated 12 years after the diagnosis. Supplementation with high-dose pyridoxine, cobalamin and folic acid lowered the total homocysteine level in plasma from 370µmol/L to 102µmol/L.The addition of betaine led to the normalization of homocysteine level. AT also was normalized after therapy (101%).
A careful review of her medical records after the diagnosis showed a personal history of depression and osteopenia, compatible with the diagnosis of homocystinuria.
Case 4
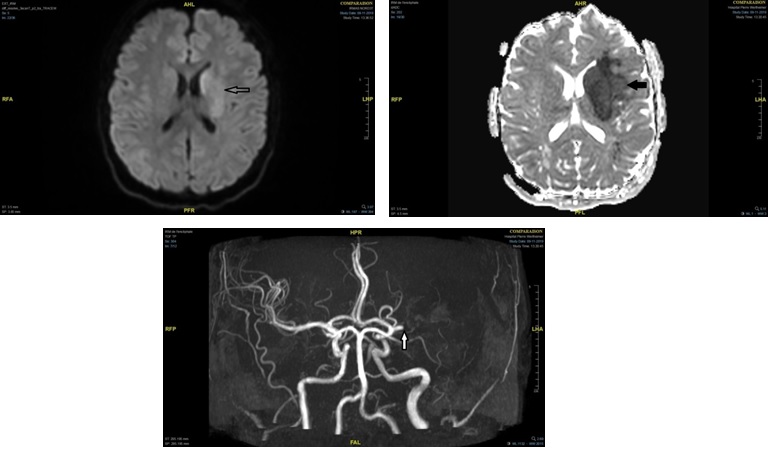
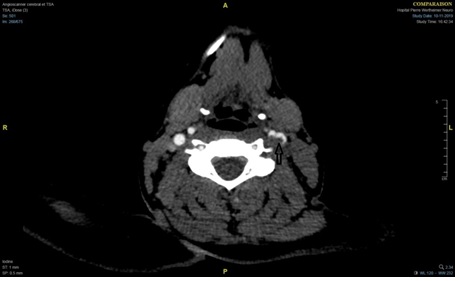
The fourth case was a 21-year-old woman with a history of venous and arterial thrombosis, but without cardiovascular risk factors. At the age of 17 years, she presented spontaneous extensive cerebral venous thrombosis (CVT) of the superior sagittal, straight and transverse sinuses, and the left internal jugular vein. Long-term anticoagulant therapy with a direct oral anticoagulant (DOAC) was prescribed. One month later, she had facial paralysis, but MRI did not confirm recurrent CVT. Thrombophilia testing, performed after a wash-out period from the DOAC, did not detect antiphospholipid syndrome, factor V Leiden mutation, and prothrombin gene mutation. There was no PS deficiency, but moderate PC and AT deficiency (Table 1). The patient also had a mild factor VII (FVII) deficiency (35%). No genetic abnormality of the PC, SERPIN C1 and F7 genes was detected. Blood cell count showed no abnormalities. Four years later, at the age of 21, while on DOAC therapy, she had a Sylvian fissure ischemic stroke treated by mechanical thrombectomy (Figure 2). An early recurrence required a new thrombectomy with partial recanalization of the left medium cerebral artery complicated by embolism in the contralateral Sylvian and cerebellum territories and hemorrhagic transformation. A floating thrombus of the left carotid sinus, extending into the internal and external carotid arteries, was identified (Figure 3). In addition, a thrombus of the right common femoral artery was incidentally found during hospitalization. Long-term anticoagulation therapy with warfarin was decided. Complementary laboratory tests showed severe homocysteinemia with very high levels of total homocysteine (308µmol/L) (Table 1). Amino acid chromatography suggested CBS deficiency that was confirmed by genetic testing showing compound heterozygosity for the CBS mutations p.Thr257Met and p.Val479Gly. Treatment with high-dose pyridoxine, cobalamin, and folic acid, associated with betaine and low-protein diet, fully normalized homocysteinemia (12µmol/L) within a few weeks.AT activity also improved (currently at 70%).
The patient had severe myopia, and after the diagnosis of homocystinuria, it was noticed that she had bilateral lens subluxation that can be associated with CBS deficiency
Discussion
The four patients described in this article present several clinical and laboratory similarities. In all patients, the first episode of thrombosis occurred before the age of 25 years, followed by regular recurrences. In one patient, recurrent VTE occurred despite prophylactic anticoagulation. Laboratory testing often reported transient AT deficiency that was improved or normalized after specific treatment. Our data are in accordance with the results of a large series of 629 patients with homocystinuria in which the risk of thrombosis was 25% before the age of 16 and increased to 50% by the age of 30 years, emphasizing the early appearance of vascular events in this disease [2]. In another cohort of patients with homocystinuria, vascular events were the symptom leading to the diagnosis in 25% of patients [5]. Thromboembolic events are also associated with mortality in almost 80% of patients [2]. Myocardial infarction, stroke, and PE are the main causes of mortality. Fortunately, specific treatment (pyridoxine, betaine, and methionine-restricted diet) to lower plasma homocysteine significantly reduces the cardiovascular risk in CBS deficiency [6]. Thus, early diagnosis is crucial to initiate treatment and prevent thrombotic complications. Among the four patients presented here, only one had arterial thrombosis. She had a stroke at the age of 21 and also presented large artery stenosis, as previously reported [7,8].
Several mechanisms have been proposed to explain the occurrence of of vascular events in patients with severe homocysteinemia, but little is known about the pathophysiology of thrombosis in this disease. In vitro studies suggest that homocysteine might inhibit the endothelium antithrombotic function, increase tissue factor expression, inhibit the activity of PC and AT, and activate platelets [7,8]. Animal studies confirmed that severe homocysteinemia can induce dysfunction of the vascular endothelium and loss of endothelium-dependent vasodilation, two key changes that increase the risk of thrombosis. Our four patients had transient AT deficiency until specific treatment lowered plasma homocysteine level to normal values. The association between AT activity and CBS deficiency has been described in previous case reports and series [9–14]. However, the mechanisms underlying AT deficiency in severe homocysteinemia are unknown. A possible direct effect of excessive circulating homocysteine or methionine was previously suggested to explain the acquired AT deficiency observed in these patients [10,15]. AT and other coagulation deficiencies induced by severe homocysteinemia may explain the hypercoagulability that increases the risk of thromboembolic events in this disease. One of our patients (case 4) had moderate AT, PC, and FVII deficiencies detected during thrombophilia testing. Homocystinuria-associated PC and FVII deficiencies have been previously described, but are less frequent than AT deficiency [10–12,16]. FVII deficiency and thrombosis are not contradictory findings because some mutations that cause FVII deficiency are associated with a high risk of thrombosis [17]. In patient 4, some family members also had mild asymptomatic FVII deficiency, suggesting the inheritance of two distinct abnormalities (CBS defect and FVII deficiency). Differently from AT deficiency, FVII or PC level improvement and normalization of plasma homocysteine levels could not be evaluated because of the long-term VKA (warfarin) treatment.
It has been demonstrated that moderate homocysteinemia and MTHFR gene mutations are not significant risk factors for VTE [18]. Therefore, plasma homocysteine is not systematically measured in young patients with venous thrombosis.
However, the detection of mild AT deficiency or combined coagulation defects, such as AT and PC deficiency, during thrombophilia screening may be a warning signal for clinicians. This should lead to a measurement of plasma homocysteine level, particularly if no SERPIN C1 gene abnormality is detected. The care pathway of these four patients highlights the lack of knowledge of this rare inherited disorder by non-specialist physicians.
Conclusion
Although CBS deficiency is a rare disorder, specific treatment can be offered to efficiently reduce plasma homocysteine levels and the incidence of thrombotic complications that are the main causes of morbidity and mortality. Such an approach is not possible without an early diagnosis. Hematologists and vascular medicine specialists should be aware of the large and unspecific spectrum of symptoms that appear in young adulthood. Careful interrogation and physical examination are essential for detecting clinical signs that may suggest homocystinuria.
Ethical approval: consent has been obtained from each participant knowledge of understanding purpose and nature of procedures for the study
- Morris AAM, Kožich V, Santra S, Andria G, Ben-Omran TIM, Chakrapani AB, et al. (2017) Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J Inherit Metab Dis. 40: 49-74.
- Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, et al. (1985) The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 37: 1-31.
- Guilland J-C, Favier A, Potier de Courcy G, Galan P, Hercberg S (2003) L’hyperhomocystéinémie: facteur de risque cardiovasculaire ou simple marqueur ?: 1. Données fondamentales. Pathol Biol. 51:101-110.
- Cozar M, Urreizti R, Vilarinho L, Grosso C, Dodelson de Kremer R, et al. (2011) Identification and functional analyses of CBS alleles in Spanish and Argentinian homocystinuria patients. Hum Mutat. 32: 835-842.
- Magner M, Krupková L, Honzík T, Zeman J, Hyánek J, Kožich V (2011) Vascular presentation of cystathionine beta-synthase deficiency in adulthood. J Inherit Metab Dis. 34: 33-37.
- Yap S, Boers GH, Wilcken B, Wilcken DE, Brenton DP, Lee PJ, et al. (2001) Vascular outcome in patients with homocystinuria due to cystathionine beta-synthase deficiency treated chronically: a multicenter observational study. Arterioscler Thromb Vasc Biol. 21: 2080-20805.
- Bos GM, den Heijer M (1998) Hyperhomocysteinemia and venous thrombosis. Semin Thromb Hemost 24: 387-391.
- den Heijer M, Keijzer MB (2001) Hyperhomocysteinemia as a risk factor for venous thrombosis. Clin Chem Lab Med. 39: 710- 713.
- Giannini Margaret J, Coleman M, Inner field I (1975) Antithrombin Activity in Homocystinuria. The Lancet. 305: 1094.
- Palareti G, Salardi S, Piazzi S, Legnani C, Poggi M, Grauso F, et al. (1986) Blood coagulation changes in homocystinuria: Effects of pyridoxine and other specific therapy. J Pediatr.109: 1001-1006.
- Schienle HW, Seitz R, Rohner I, Lerch L, Krumpholz B, Krauss G, et al. (1994) Coagulation factors and markers of activation of coagulation in homocystinuria (HOCY): a study in two siblings. Blood Coagul Fibrinolysis Int J Haemost Thromb 5: 873-878.
- Kang HS, Kim DG, Yoon BW (1998) Superior sagittal sinus thrombosis with homocystinuria and deficiency of antithrombin III and factor VII: case report. Acta Neurochir (Wien) 140: 196- 198.
- Hong HS, Lee HK, Kwon KH (1997) Homocystinuria presenting with portal vein thrombosis and pancreatic pseudocyst: a case report. Pediatr Radiol 27: 802-804.
- Maruyama I, Fukuda R, Kazama M, Abe T, Yoshida Y (1977) [A case of homocystinuria with low antithrombin activity (author’s transl)]. Nihon Ketsueki Gakkai Zasshi J Jpn Haematol Soc. 40: 267-271.
- Mudd SH (1979) Diseases of sulfur metabolism: implications for the methionine-homocysteine cycle, and vitamin responsiveness. Ciba Found Symp 239-258.
- Munnich A, Saudubray J-M, Dautzenberg M-D, Parvy P, et al. (1983) Diet-responsive proconvertin (factor VII) deficiency in homocystinuria. J Pediatr. 102: 730-734.
- Mariani G, Herrmann FH, Schulman S, Batorova A, Wulff K, Etro D, et al. (2003) Thrombosis in inherited factor VII deficiency. J Thromb Haemost JTH. 1: 2153-2158.
- Lijfering WM, Coppens M, Veeger NJGM, Middeldorp S, et al. (2008) Hyperhomocysteinemia is not a risk factor for venous and arterial thrombosis and is associated with elevated factor VIII levels. Thromb Res. 123: 244-250
 Figure 1
Figure 1
 Figure 2
Figure 2
 Figure 3
Figure 3

Tables at a glance