Use of Enhanced Recovery after Surgery (ERAS) Protocol for Immediate Sub-Muscular Breast Reconstruction after Outpatient Mastectomy Is Safe and Significantly Reduces Health System Costs
Received Date: January 27, 2020 Accepted Date: February 27, 2021 Published Date: March 01, 2021
doi: 10.17303/jcrto.2021.9.104
Citation: Ryan Kunkel (2021) Use of Enhanced Recovery after Surgery (ERAS) Protocol for Immediate Sub-Muscular Breast Reconstruction after Outpatient Mastectomy Is Safe and Significantly Reduces Health System Costs. J Cancer Res Therap Oncol 9: 1-7.
Abstract
Background: Enhanced recovery after surgery (ERAS) protocols are associated with decreased postoperative stays, reduced opioid use, lower rates of postoperative nausea and vomiting, and lower overall costs to institutions and healthcare systems. The aim of this study was to evaluate the impact of an ERAS approach on mastectomy with implant-based subpectoral reconstruction (IBR) with respect to procedure costs and 30-day complication rates for both ambulatory surgery patients and patients hospitalized overnight.
Methods: We retrospectively compared the outcomes of 63 consecutive patients following nipple-sparing mastectomy or skin-sparing mastectomy in either an ERAS vs. overnight stay (OVS) cohort during the period 2014-2016 at an academic center. Demographics, comorbidities, 30-day complications, and cost analyses were examined.
Results: A total of 63 distinct patients underwent 68 surgical encounters for a total of 102 breasts excised. There were 34 encounters in each group. Statistical differences between the cohorts were observed only with BMI and social barriers to health care, p=0.04 and p=0.02 respectively. Thirty-day postoperative complication rate differences did not reach statistical significance, (p=0.2). Cost data demonstrated a statistically significant reduction in both direct (95% CI: $2307-$8,658; p=0.001) and indirect costs (95% CI: $676-$2580; p=0.0013) in the ERAS cohort compared to patients admitted overnight.
Conclusions: There were no significant differences in 30-day complication rates between ERAS and OVS groups. Social barriers were the principal determinants of disposition. A 30% cost savings was seen with application of ERAS principles and a same-day surgery approach.
Keywords: ERAS, Breast Reconstruction, Cost Analysis
List of Abbreviations: ERAS: Enhanced Recovery After Surgery; IBR: Implant-based subpectoral reconstruction; OVS: overnight stay; NSM: Nipple-sparing mastectomy; SSM: Skin-sparing mastectomy; BMI: Body mass index; OSA: Obstructive sleep apnea; DM: Diabetes mellitus; CAD: Coronary artery disease; CKD: Chronic kidney disease stage 3 or greater; PACU: Post-anesthesia care unit
Introduction
US Cancer Statistics (2021) estimate 280,550 new annual cases of breast cancer in women [1]. Over the last decade, studies have demonstrated an increase in contralateral prophylactic mastectomies and a decrease in breast conservation procedures [2-7]. Tuttle and colleagues documented a near tripling of contralateral prophylactic mastectomies in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database over a 5-year period [8]. Receipt of bilateral mastectomy increased from 3% in 1998 to 18% in 2007, according to a Market Scan database report [9].
While rates of mastectomies are increasing, there is a shift towards implant-based reconstruction (IBR) and away from autologous reconstruction [6,7,9,10]. One study reported a near doubling of the rate of IBR over a 5-year span [6]. Another study found that IBR increased an average of 11% per year between the years 1998-2008, while rates of autologous reconstructions did not change [11].
Historically, patients have been admitted to the hospital overnight for pain control and monitoring following major breast surgery. Retrospective studies have shown no increase in complications when mastectomy alone is performed on an outpatient basis [12]. Studies on Enhanced Recovery After Surgery (ERAS) protocols have documented shorter postoperative stays, reduced opioid use, lower rates of postoperative nausea and vomiting, and lower overall costs to institutions and healthcare systems. ERAS protocols associated with urologic and colorectal cancer surgery generated much of the early literature [13,14]. Following these successes, other specialists have utilized ERAS concepts including hepatobiliary, bariatric, and pancreatic surgeons [15-17].
There is increasing interest in the application of ERAS protocols to breast surgery [18,19]. A recent search of “ERAS protocols in breast surgery” failed to identify any randomized trials or cost analysis using actual patient and facility expense data. Studies evaluating cost lack uniformity in both the manner and type of information collected which have resulted in a wide range of reported outcomes [20]. However, there is general agreement that ERAS leads to lower costs primarily due to reduced postoperative management expenses, which include reduced hospital stay and decreased use of ancillary services [21].
The aim of this study was to evaluate the impact of an ERAS approach on implant-based subpectoral breast reconstruction with respect to procedure costs and 30-day complication rates for both ambulatory surgery patients and patients hospitalized overnight or longer. We specifically chose our initial ERAS transition cohort, with sub pectoral positioning of implants, a location expected to be more “pain-generating” compared to our current practice of pre pectoral implant positioning, to assess for applicability of ERAS protocols.
Methods
This study was approved by the Institutional Review Board at the University of New Mexico Hospital. A retrospective review of female patients over the age of 18 years who underwent a nipple-sparing mastectomy (NSM) or skin-sparing mastectomy (SSM) with subpectoral IBR from 2014-2016 was conducted. Patients were excluded if they had autologous reconstruction or if implants were placed in a pre-pectoral plane. A total of 70 surgical encounters met these criteria.
All cases were performed at facilities associated with the University of New Mexico. Two breast surgeons and three plastic surgeons performed all operations and were familiar with the ERAS protocol. Patients were separated into two cohorts: ERAS and Overnight Stay (OVS). The ERAS cohort consisted of patients who were scheduled for same-day surgery at an ambulatory surgical center (ASC). Patients were screened preoperatively for major comorbidities and/or social factors that would preclude their ability to discharge on the same day as surgery. The ERAS protocol was developed based on both surgeon preference and published data. ERAS elements included preoperative education, advance provision of prescriptions, and preoperative use of paravertebral or erector spinae nerve block.Narcotic analgesics were allowed, but minimized.. In the recovery phase, patients were expected to use scheduled non-steroidal anti-inflammatory medication and acetaminophen.
Data collection was performed using the hospital’s electronic medical record system. Age and comorbidities that included body mass index (BMI), obstructive sleep apnea (OSA), diabetes mellitus (DM), coronary artery disease (CAD), and chronic kidney disease stage 3 or greater (CKD) were recorded. Social factors were defined as either having insufficient family support for same-day discharge or being located further than 50 miles from care. These barriers were assessed by the operating breast surgeon and documented in preoperative consultation notes. Surgical indication (therapeutic vs. prophylactic) and type of mastectomy (NSM vs. SSM) were compared. Complications were defined as the occurrence of at least one of the following: infection, hematoma or hemorrhage, nipple or skin flap necrosis, urinary tract infection, expander loss, return to the operating room within 30 days, and systemic complications such as thromboembolism, or cardiopulmonary events. Any Emergency Department (ED) visit within 30 days of surgery was noted. Length of hospitalization, mean operative time, and mean time in post-anesthesia care unit (PACU) were determined.
Cost data was retrieved using the hospital accounting systems. Direct costs were defined as patient-specific costs, such as pharmacy and operating room charges. Indirect costs included a facility-derived multiplier added to the encounter to cover fixed expenses such as salaried labor and building overhead. Mean, (standard deviation, SD), median and 95% confidence interval (95% CI) were calculated for continuous variables. Indirect, direct, and total costs were compared by type of admission (OVS vs. ERAS) and laterality (unilateral vs. bilateral). We used two-sided t-test for normally distributed data, exact Pearson’s Chi Square test for categorical data, and one-way ANOVA for multiple group comparisons. A reported p-value <0.05 was considered statistically significant. All analyses were performed using SAS software version 9.4 (SAS Inc., Cary, NC).
Results
Four hundred and ninety-four patients underwent 742 mastectomies from 2014-2016. We excluded those patients not receiving reconstruction and those receiving autologous reconstruction or pre-pectoral implant placement. Two patients were excluded from our analysis due to incomplete accounting records. We analyzed a total of 68 cases of NSM and SSM with subpectoral IBR. Thirty-four patients underwent bilateral mastectomy with IBR and 24 patients underwent a single unilateral mastectomy with IBR. Five patients underwent two separate unilateral mastectomies with IBR, which were treated as 10 unique cases. In total, 63 distinct patients underwent 68 surgical encounters for a total of 102 breasts excised.
The ERAS group included 34 surgical events, 17 were unilateral mastectomies and 17 bilateral; a total of 51 breasts were excised. The OVS group included 34 surgical events; 17 unilateral, 17 bilateral, and a total of 51 breasts excised. The average age of patients in the ERAS group was 45.7 years and average BMI was 25.3 kg/m2. One patient had DM (2.9%); 4 patients had OSA (11.8%); and no patients had CAD, CKD, or clinically significant social factors. The average age in the OVS group was 49.0 years and average BMI was 27.8 kg/m2. Two patients had DM (5.7%); 1 patient had OSA (2.9%); and no patients had CAD or CKD. Six patients (17.6%) had social factors impacting ability to discharge on the day of surgery. Statistical differences between the cohorts were observed only with BMI and social factors, p=0.042 and p=0.025, respectively (Table 1).
Surgical indications and type of mastectomy are reported by breast. In the ERAS group, 43% of total breasts were removed for active disease; 57% of breasts were removed prophylactically. NSM was utilized to remove 49/51 breasts and SSM for 2/51 breasts. In the OVS group, 55% of breasts were removed for active disease; 45% were removed prophylactically. Within this cohort, NSM was utilized for removal of 45/51 breasts and SSM for 6/51 breasts. Indications and type of mastectomy were analyzed using chi-squared test and were nonsignificant (p=0.24 and p=0.27, respectively) (Table 1).
In the ERAS cohort, 3% developed post-surgical cellulitis. In the OVS group, 6% developed post-surgical cellulitis. Three (9%) patients in the OVS group developed postoperative hematoma; one was identified in the PACU and required immediate reoperation, another was identified after the patient presented to the emergency department for increased postoperative pain and was offered a surgical evacuation and the third was managed conservatively with periodic follow-up appointments. There were no instances of venous thromboembolism, implant loss, nipple loss, or wound dehiscence in either group. The rate of complications between the two cohorts was nonsignificant, p=0.197 (Table 1).
The median length of stay in the OVS group was 1 night; 30/34 encounters (88%) required a 1-night hospitalization; 1/34 (3%) a 2-night stay; 2/34 (9%) a 3-night stay, and 1/34 (3%) a 4-night stay. All patients who stayed longer than one night required additional pain control. Three patients in the OVS cohort visited the emergency department within 30 days of surgery. One for chest wall pain and discharged home, one for increased postoperative pain secondary to a postoperative hematoma and the third for a surgical site infection. One patient in the ERAS cohort was evaluated in the emergency department one day after surgery for an unrelated motor vehicle accident and not included in our statistical analysis (Table 1).
The mean operative and PACU time for the ERAS cohort was 4 hours 29 minutes and 2 hours 30 minutes, respectively. The mean operative and PACU time for the OVS cohort was 4 hours 49 minutes and 2 hours 39 minutes, respectively. Differences between the ERAS and OVS mean operative and PACU times were nonsignificant, p=0.29 and p=0.66, respectively (Table 1).
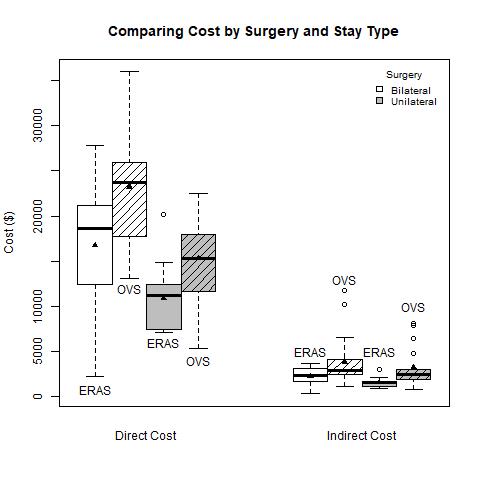
Mean direct cost was $13,791 in the ERAS cohort and $19,273 in the OVS, a difference of $5,482 (95% CI: $2307, $8,658, p=0.001). Mean indirect costs for ERAS and OVS was $1,882 and $3,511, respectively, a difference of $1,628 (95% CI: $676, $2,580, p=0.0013). Figure 1 shows stratified patients in the OVS group who underwent a bilateral operation incurred the highest mean direct cost ($27,049), whereas individuals in the ERAS group who had a unilateral surgery recorded the lowest ($12,405), p<.0001. Indirect costs show similar trends, p=0.0054 (Table 2).
In 2014, 77.8% of mastectomies with subpectoral IBR cases were performed in the hospital and 22.2% were performed in an ambulatory surgical center utilizing the ERAS protocol. In 2015, the percentages were 52.5% and 47.5%, respectively. By 2016, 19.0% were performed in the hospital and 81.0% in an outpatient center with the ERAS protocol.
Discussion
The aim of enhanced recovery protocols is to minimize the stress of surgery on the patient using evidence-based guidelines concurrent with the end points of multidisciplinary management. Global literature cites decreased morbidity and length of stay as the two most frequently reported clinical benefits [22,23]. Cost benefits have also been the subject of study across a variety of surgical subspecialties within an ERAS framework [24,25]. The method by which cost reduction is achieved is not uniform. For example, Pache and associates reporting an ERAS cost analysis for gynecologic procedures concluded cost savings were generated by reduced pre- and post -operative care elements. These elements were statistically significant when bundled, with reduced nursing care costs appearing to drive the savings. Intraoperative and anesthesia costs were not statistically different [28]. A cost analysis published by Joliat and colleagues reviewed ERAS and pre-ERAS hepatic procedures and found a nonsignificant reduction in total mean charges. Nevertheless, that study showed a significant decrease in complications and length of stay favoring ERAS patients, 49% and 8 days vs. 64% and 10 days, respectively [27]. A second study by the same investigators reviewing cost benefits in the setting of pancreaticoduodenectomy yielded similar findings, with cost decrements seen in the areas of medication charges, fewer intensive care unit admissions, and anesthesia charges; however, it did not find statistically significant total cost differences between the two groups [25]. It is unclear why these significant elements in the cost tabulation did not have a greater total cost reduction impact. A study conducted in Alberta, Canada focusing on colorectal procedures demonstrated an estimated reduction in per patient costs ranging between $2,806 and $5,898 USD as a result of ERAS implementation. The authors attributed the cost savings to decreased length of stay (2.3 days) and reduced readmissions secondary to complications (RR 1.73) [28]. Their cost estimates were not based on actual cost data accessed from patient charts, unlike the present study.
The current study validates the safety of performing same-day mastectomy with subpectoral reconstruction on an outpatient basis utilizing an ERAS protocol. The two cohorts in our study were similar with respect to comorbidities, type of mastectomy, and surgical indication. All patients in the ERAS group were discharged the day of surgery. There were no procedure-related 30-day ED encounters for the ERAS group. During our investigational period, we transitioned from a primarily hospital-based approach to an ambulatory care process. We confirmed a significant decrease in both direct and indirect costs when utilizing an ERAS protocol in an ambulatory setting compared to hospital-based surgery. A mean total cost reduction (direct and indirect) of 30% was calculated in favor of the ERAS cohort. While a direct comparison of costs between a pre-ERAS period and the study period was not performed, the cost reduction seen in this study is a result of the absence of post-operative admission expenses.
Our study has a number of strengths including applicability to most institutions. Our protocol is simple and does not require excessive resources such as an ERAS nurse coordinator. Moreover, our patient population was highly selected and well suited to undergo multiple procedures, a likely denominator for most breast reconstruction programs. Furthermore, cost data were collected from a closed system during a relatively short time frame, thereby allowing for reliable comparisons and eliminating the need for cost adjustments.
There are several limitations to this study. First, since our sample size consisted of fewer than 100 patients, we were unable to determine if a specific demographic variable or set of variables, other than social barriers, could predict the need for admission. A larger cohort might reveal different patterns associated with admission requirements.
Second, our analysis did not include patient-reported outcomes. A study by Dumestre and colleagues evaluated the impact of ERAS on “quality of recovery” from the patient perspective after individuals underwent mastectomy with implant-based reconstruction. Patients in the ERAS cohort reported increased levels of “good sleep” and “well-being” while experiencing less “severe pain” compared to peers in the traditional recovery and transition group [19]. We did not prospectively evaluate pain scores in our study. Our program transitioned to a pre-pectoral implant location following 2016. We believe the selected study group, specifically those with a sub-pectoral implant location, maximized the feasibility of a same-day discharge concept.
We included social environment as an independent variable, and with the exception of distance to home, was subjectively assessed. Social environment reached statistical significance in our study, but the wider clinical implications of this observation are unknown. Finally, since the present investigation includes a transition period, patient preference regarding stay type influenced cohort designation in the early phase.
Conclusion
There were no significant differences in 30-day complication rates between patients whose mastectomy with reconstruction procedure was at an ambulatory surgical center, utilizing our ERAS protocol, and patients whose operation was at a hospital facility with a planned overnight admission. Social factors were the main determinant for identifying those patients who could be safely discharged from an ambulatory setting. On average, a 30% cost saving can be expected with the application of ERAS principles and a same-day surgery approach.
In summery, the ERAS protocols for immediate sub-muscular implant based breast reconstruction after unilateral or bilateral mastectomy is both safe and significantly reduces health system costs. Future studies will likely confirm the advantages of the ERAS protocol as applied to breast reconstruction patients as more facilities adopt cost-based approaches. We advocate strongly for widespread implementation in hospitals.
- US Cancer Statistics Atlanta (GA) (2021) Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute.
- Kwok A, Goodwin I, Ying J (2015) National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. The Am J Surg 210: 512-6.
- Stucky C, Gray RJ, Wasif N, et al (2010) Increase in contralateral prophylactic mastectomy: echoes of a bygone era? Surgical trends for unilateral breast cancer. Ann Surg Oncol 17: 330-7.
- McGuire K, Santillan A, Kaur P (2009) Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 16: 2682-90.
- Jones N, Wilson J, Kotur L (2009) Contralateral prophylactic mastectomy for unilateral breast cancer: an increasing trend at a single institution. Ann Surg Oncol 16: 2691-6.
- Fitzpatrick A, Gao L, Smith B (2014) Cost and outcome analysis of breast reconstruction paradigm shift. Ann Plast Surg 73: 141-9.
- Boughey J, Schilz S, Van Houten H (2017) Contralateral prophylactic mastectomy with immediate breast reconstruction increases healthcare utilization and cost. Ann Surg Oncol 24: 2957-64.
- Tuttle T, Habermann E, Grund E (2007) Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 25: 5203-9.
- Jagsi R, Momoh A, Alderman A (2014) Trends and variation in use of reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol 32: 919-26.
- Liu D (2017) New plastic surgery statistic and breast reconstruction trends. American Cancer Society.
- Albornoz CR, Bach PB, Mehrara BJ (2013) A paradigm shift in U.S. breast reconstruction: increasing implant rates. Plast Reconstr Surg 131: 15-23.
- Marchal F, Dravet F, Classe J (2005) Post-operative care and patient satisfaction after ambulatory surgery for breast cancer patients. EJSO 31: 495-9.
- Arumainayagam N, McGrath J, Jefferson K (2008) Introduction of an enhanced recovery protocol for radical cystectomy. BJU Int 101: 698-701.
- Roulin D, Donadini A, Gander S (2013) Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg 100: 1108-14.
- Hall TC, Dennison AR, Bilku DK (2012) Enhanced recovery programs in hepatobiliary and pancreatic surgery: a systematic review. Ann R Coll Surg Engl 94: 318-26.
- Mannaerts G, Van Mil S, Stepaniak P (2015) Results of implementing an enhanced recovery after bariatric surgery (ERABS) protocol. Obes Surg 26: 303–12.
- Morgan K, Lancaster W, Walters M, et al (2015) Enhanced recovery after surgery protocols are valuable in pancreas surgery patients. J Am Coll Surg 222: 658-64.
- Arsalani-Zadeh R, ELFadl D, MacFie Y (2011) Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg 98: 181-96.
- Dumestre D, Redwood J, Webb C (2017) Enhanced recovery after surgery (ERAS) protocol enables safe same-day discharge after alloplastic breast reconstruction. Plast Surg 25: 249-54.
- Lemanu D, Singh P, Stowers J, et al (2014) A systematic review to assess cost effectiveness of enhanced recovery after surgery programmes in colorectal surgery. Colorectal Dis 16: 338-46.
- Kariv Y, Delaney C, Senagore A, et al (2007) Clinical outcomes and cost analysis of a “fast track” postoperative care pathway for ileal pouch-anal anastomosis. A case control study. Dis Colon Rectum 50: 137-46.
- Greco M, Capretti G, Beretta L (2014) Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 38: 1531-41.
- Ljungqvist O, Scott M, Fearon K (2017) Enhanced recovery after surgery: a review. JAMA Surg. 152: 292-8.
- Smith T, Wang X, Singer M (2020) Enhanced recovery after surgery: a clinical review of implementation across multiple surgical subspecialties. Am J Surg 219: 530-4.
- Joliat G, Labgaa I, Petermann D (2015) Cost–benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg 102: 1676-83.
- Pache B, Joliat G, Hubner M (2019) Cost-analysis of enhanced recovery after surgery (ERAS) program in gynecologic surgery. Gynecol Oncol 54: 388-93.
- Joliat G, Labgaa I, Hübner M (2016) Cost-benefit analysis of the implementation of an enhanced recovery program in liver surgery. World J Surg 40: 2441-50.
- Nelson G, Kiyang L, Crumley E, et al (2016) Implementation of enhanced recovery after surgery (ERAS) across a provincial healthcare system: the ERAS Alberta colorectal surgery experience. World J Surg. 40: 1092-103.

Tables at a glance
Figures at a glance