Human Granulosa Cells, A New Type of Stem Cells, Can Differentiate into Sperm-Like Cells
Received Date: November 20, 2020 Accepted Date: December 28, 2020 Published Date: December 30, 2020
doi: 10.17303/jscr.2020.2.102
Citation: Elham Ghadirkhomi, Masoud Maleki, Mohammad Reza Mashayekhi (2020) Granulosa Cells, A New Type of Stem Cells, Can Differentiate into Sperm-Like Cells. J Stem Cell Rep. 1: 1-10.
Abstract
It was proposed that granulosa cells arise from the population of stem cells during follicular growth. According to previous studies human-derived luteal phase granulosa cells are able to differentiate into other cell lineages, such as osteoblasts, chondrocytes and neurons. In this study we investigated the differentiation potential of follicular-phase granulosa cells into sperm-like cells. First, we demonstrated the stemness of isolated GCs by flowcytometry and then we showed that granulosa cells can differentiate into advanced male germ cell lineages including spermatid-like cells in vitro. Follicular-phase GCs were cultured then induced by sheep testis tissue extract to differentiate into sperm -like cells , as evidenced by their expression of Gpr125, as well as VASA and DAZL, which are markers of different steps of Spermatogenesis. Mesenchymal stem cell markers were expressed in these cells. Spermatogenesis markers, DAZL, VASA and GPR125, were also expressed from the second week after induction. Morphological analysis of induced cells showed that Producing sperm- like cells were similar to the normal sperm. Obtained results prove that isolated GCs are a kind of mesenchymal stem cells and have the capability to differentiate into sperm – like cells that may represent a novel strategy for studying spermatogenesis in vitro.
Keywords: Mesenchymal Stem Cells; Granulosa Cells; Spermatogenesis; VASA; Gpr125; DAZL
Introduction
Stem Cells
Stem cells are undifferentiated cells capable of self-renewing and differentiating into various types of cells in adult tissues influenced by proper molecular signals [1]. In mammals based on the origin of stem cells, these cells are categorized into three groups: embryonic stem cells, embryonic germ cells and adult stem cells [2]. Mesenchymal stem cells are a type of adult stem cell that because of some unique properties, are interested in cell therapy applications of regenerative medicine [3]. The main reasons of interest toward these cells are that mesenchymal stem cells can be isolated from various sources such as adipose tissue, peripheral blood, umbilical cord and placenta, and their ability to expand rapidly in vitro which let them reach the number of cells proper for cell therapy easily and quickly [4].
Granulosa Cells
Granulosa cells (GCs) are a type of somatic cells surrounding the growing follicles. These cells remain very close to the basement membrane and are essential for the production of estrogen and ovulation [5,6]. GCs have several specific functions including secretion of large amounts of hormones, response to FSH hormone (follicle stimulating hormone) and leuteinizing hormone (LH) and delivery of the follicle [5]. According to the previous studies, GCs have properties of stem cells [7]. GCs can survive for long periods when cultured in vitro in the presence of leukemia inhibitory factor (LIF). After culture of them, they lack the activity of follicle-stimulating hormone receptors and aromatase enzyme gradually while Oct-4, which is a common marker of stem cells, begins to express in them [8].
It has been detected that telomerase enzyme, which has activity in maintenance of chromosome ends during cell division, was expressed in pig ovary [9]. Meanwhile, multipotency of GCs has been proved by differentiating these cells into other types of cells, such as neurons, chondrocytes, and osteoblasts in vitro [8]. Some other evidences indicated that in animal cloning experiments, GCs have been used as nuclei donors [10,11]. Varras, et al. [12] showed expression of Oct-4 in GCs by analyzing of oct-4 mRNA and then these cells are multipotent stem cells [12].
Granulosa Cells
To produce a functional sperm, male germ cells undergo a series of processes which have collectively named spermatogenesis [13]. 123×106 sperms are produced daily in seminiferous tubules of testis [14,15]. Clermont for the first time identified spermatogonia type Adark and Apale in human testis, and demonstrated that spermatogonia type Adark acts as reserve stem cells while spermatogonia type Apale acts as renewing stem cells [16-18]. It is suggested that spermatogonia type A inside seminiferous tubules divides every 16 days and differentiates into spermatogonia type B which then in a mitotic division, coverts to primary spermatocyte [18]. Spermatocytes convert to haploid spermatids after meiosis and then transform into mature spermatozoa which this process takes 64 days in human [19,20]. Immunohistochemistry results show that GPR125 (Gprotein- coupled receptor 125) is expressed in a subset of spermatogonia type A cells, and is used as a marker for mouse spermatogonia and progeny [21,22].
When cells enter meiosis, in male and female gonocytes DAZL protein and VASA transcripts are expressed and the expression of Oct-3/4 decreases [23]. DAZL protein is an RNA-binding protein that belongs to DAZL family including DAZ and BOULE. DAZL gene family code proteins with conserved RNA binding motifs and DAZ sequence of 24 amino acids. It is thought that these proteins are involved in post-transcriptional regulation of mRNA [24,25]. In men during spermatogenesis DAZL express in gonocyte, spermatogonia and primary spermatocytes. As meiosis progress, DAZL can be exited from the nucleus into cytoplasm of spermatogonia, secondary spermatocytes, spermatids and spermatozoa [25-28]. Reduction of DAZL expression can be seen in human testes which have low or no sperm production [26].
DAZL protein expression has been reported in human and rat granulosa cells [26,27] human teca cells [29] and luteal granulose cells [26,30]. By entering the genital ridge, germ cells express another specific marker named VASA, which is a cytoplasmic protein involved in the regulation of protein translation. DDX4, encode VASA, is evolutionary conserved and studies show that VASA protein has an important role in the function of germinal layer [31]. Synaptonemal complex proteins (SCPs) including SCP1, SCP2, and SCP3 are important in synapsis construction in meiosis and are meiosis markers [32]. At the final stages of spermatogenesis TEKT1 expression was detected [23].
Aim and Importance
15% of couples have infertility, that male factors contribute 40%–60% of cases of infertility [33]. Azoospermia, some men are affected, is a condition in which there is no sperm in semen sample . This condition affects about 1% of the men and may cause 20% of male infertility. Nayernia, et al. [34] reported differentiation of murine embryonic stem cells (ESCs) into male gametes and injected these sperm- like cells into oocytes that resulted viable mice [34]. Clark, et al. [35] evaluated potency of human ESCs to production of germ cells. They established that differentiated cells have specific markers of germ line development such as DDX4, DAZL by using PCR and immunohistochemistry.
Furthermore, meiotic marker gene, SYCP3, was detected in differentiated cells. Therefore, this study resulted that human ESCs of both sexes can enter in germ line development pathway [35]. For men that have a problem in sperm producing without a genetic cause, stem cell transplantation thought to be the proper method [36]. Some studies show that human pluripotent stem cells (hPSCs) have the capacity to enter meiosis and result haploid cells [37]. In current study we have investigated stemness of the GCs and their ability to differentiate into sperm- like cells.
Materials and Methods
Culture and Confirming the Nature of Granulosa Cells as Mesenchymal Stem Cells
Isolated GCs were cultured and identified mesenchymal stem cell surface markers as described previously [38]. Follicles were collected by aspiration of infertile women’s ovaries according to assisted reproductive technology (ART). Then GCs that isolated enzymatically, were cultured in T25 cell culture flasks (orange scientific, Belgium) containing DMEM/F12 supplemented with 20% FBS and 1%penicillin and streptomycin and incubated at 37 oC and 5% CO2 in humidifier incubator. Then GCs analysed by flow cytometry for mesenchymal stem cell markers (R&D system, USA) including anti- Stro-1, anti- CD90, anti- CD105, anti- CD106, anti-CD146, anti- CD166, anti- CD44, anti-CD45 and anti- CD19 anti-bodies.
Preparing Sheep Testis Extract
Sheep testicles transferred under hood after alcohol spray, epididymis and external part of testes was cut and completely removed. Testes were weighed then testicular tissue was cut into tiny pieces using a surgical blade and mixer. For every 16.5 gr of testicular tissue, approximately 5 ml of HBSS was added. The mixture was transferred to a 50 mL falcon and centrifuged for 10 minutes at 4400 rpm. Supernatant was transferred to a 15 mL falcon and sediment discarded. This step was repeated three times. The last supernatant was collected, passed through a filter paper. 1 ml antibiotic Pen / Strep 100X was added per 12 ml of collected extract. The obtained extract was transferred to a 1.5 cc micro-tubes and was ultra-centrifuged for 5 minutes with 14000 rpm. Supernatants were transferred to new micro-tubes and the precipitates discarded. Two last steps were repeated three times. Obtained extracts were determined for concentration of testosterone. Remained extracts were stored at -20ºc. Extracts in which testosterone level was 4- 7ng/ ml was used as inducer.
Investigating the Ability of Human Granulosa Cells Differentiation into Sperm Cell Lineage
Cultures that had reached more than 80% of confluence were selected for induction. Medium inside the flask was completely removed and about 5 ml of fresh induction medium was filtered into it. The composition of the induction culture medium was obtained by adding DMEM / F12, 20% FBS, 1% Pen / Strep and 30% of sheep testicular extracts. Then flasks were transferred into the CO2 incubator, and medium was changed every three days. Induced flasks were evaluated in four groups: Groups were categorized by culturing granulosa cells in the induction medium for 7days, 14 days, 21 days, and 28 days respectively. Then cell extracts were prepared and analyzed by western blotting method. One flask of each group was stained using the Diff Quick kit (Diff Quick, Avicenna institute Production Catalog No. ARI-And-01) for morphological analysis.
Morphological Study of Differentiated Cells using Sperm Quick Staining Kit (Diff Quick)
Staining methods in evaluation of sperm quality and sperm health are apparent. In the present study to compare the morphology of differentiated cells with normal sperm shape, the sperm quick staining kit was used. After the end of considered induction period for each group, one flask from each group was stained according to the kit instructions, and cells were studied under the microscope.
Western Blotting
A total of 50 μg of total protein extract was loaded in each well and subjected to SDS- page electrophoresis. Separated proteins were transferred onto nitrocellulose membranes and the membranes were probed by one of primary antibodies: GPR125 (P-15): sc-164512, VASA Antibody (H-80) sc-67185, DAZL (H-90): sc-366304 all from Santa Cruze Co. in the ration of 1/1000 . Cruz marker molecular weight standards (sc-2035 santacruze Co.) was used as protein size markers. The murine testis extract was used as positive control and non-induced granulosa cells extract was used as negative control. Detection of proteins was achieved by using secondary antibodies (goat anti- rabbit IgG-HRP: SC2004 and donkey anti-goat IgG-HRP: SC2020) with the ration of 1/7000. In order to compare protein expression in different weeks of induction, image J software was used.
Results
Cell Culture Results
Granulosa cells after isolation and during the first day of culture were roughly spherical shape, but gradually attached to the plastic culture flask and, began to cytoplasmic extension that get the fibroblastic- like shape after 7 days. (Figure 1A) cell line prepared after cell volume reach to about 80% of confluent (Figure 1B).
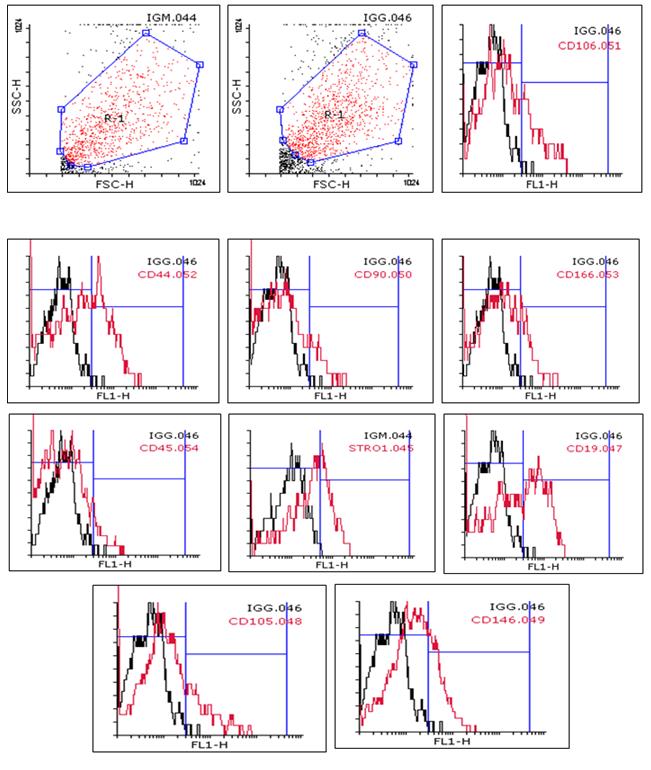
Flow Cytometry Analysis Results
After cell gating (R region), and use of control isotopes to remove cellular debris fragments, the stem cells had a different percentage of expression of each surface marker. The results showed that the percentage of cell markers Stro-1, CD19, CD105, CD146, CD90, CD106, CD44, CD166 and CD45 are respectively 34.08, 41.99, 21.47, 26.82, 13.47, 25.73, 35.90, 20.23 and 8.84 (Figure 2).
Induction Experimental Results
Morphological analysis of four experimental groups showed that, during the first week of induction, cells began to lose their fibroblastic- like shape. Nucleus of some cells was placed in the middle of the cell and two extensions on both sides of the cell were observed (Figure 3A). During the second week of induction, in each of differentiated cells, the nucleus was completely drawn into one side of the cell and a small extension was observed on the other side of the cell. The cell was formed almost like a club (Figure 3B). At the end of the third week the extension at the end of the cell was longer, head of the cell also became longer, get a short neck and the nucleus of the cell was placed in the middle of it (Figure 3C). At the end of induction time, 28 days culturing cells in sperm inducing medium, tail and head got more clear and cells were more similar to sperm morphology (Figure 3D).
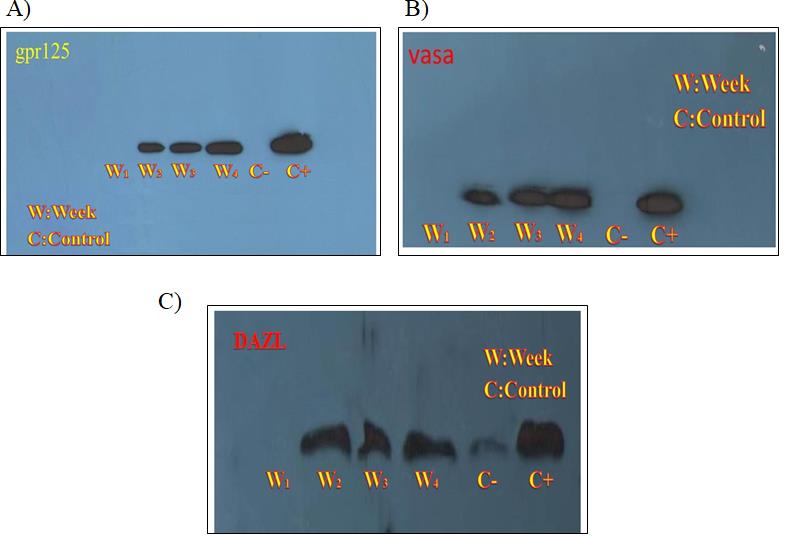
Western Blotting Analysis
Western blotting results of GPR125 protein that was used as a marker protein for spermatogonial cells, showed no expression of this protein in the first week of culture in terms of induction, but expression started from the second week of induction and showed a slight increase in the next weeks (Figure 4A). Results for two other proteins, VASA (Figure 4B) and DAZL (Figure 4C), were similar. In the control group GCs without treatment for induction were used as negative control and, testis extract was considered as positive control.
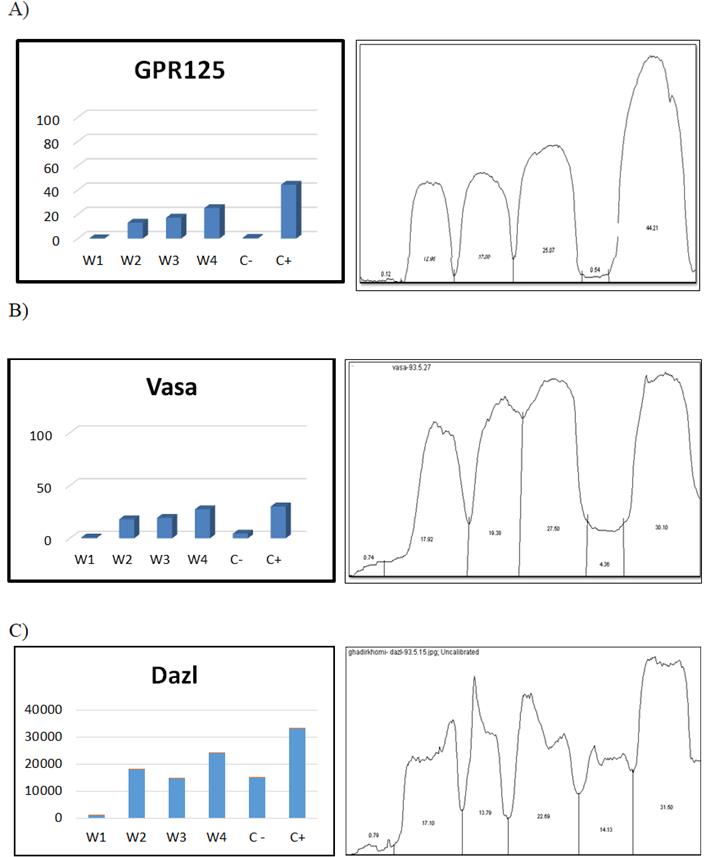
Image J Software Analysis
In order to compare documents obtained by Western blotting, Image J software was used. Comparison of protein expression in different weeks showed that the expression of GPR125 protein during four weeks was 0.125%, 12.96%, 17.08%, 25.07% respectively (Figure 5A). And the VASA protein expression in 4 different times was respectively 0.73%, 17.924%, 19.378%, 27.501% (Figure 5B). The results for DAZL protein were 0.79, 17.10, 13.79, 22.69 respectively (Figure 5C). Numbers do not indicate the exact expression level of proteins and the basis for comparison between weeks in this software was considered the widths of the bands obtained from the western blotting analysis.
Discussion
The importance of spermatogenesis process and its possible clinical application in the treatment of infertility, made many scientists to try to produce sperm in vitro. Although sperm production in men is a natural process in which various numbers of somatic cells are involved, producing healthy and functional sperm in vitro has been a goal of many scientists for about a century. There were two reports on the differentiation of immature germ cells into haploid cells in 1990 [39,40]. Later in the 2002, it was reported that mouse spermatogonia typeA cells are able to differentiate up to haploid spermatid in the presence of stem cell factor (SCF) [41]. However, some undifferentiated germ cells were observed and there was no evidence about fertilization and embryo production ability of these cells. In humans have been reported that Cells isolated from human testicular biopsy with premeiotic arrest are able to differentiate to haploid germ cells when cultured for 48 hours [42]. These in vitro produced spermatids significantly showed conception ability and led to formation of a healthy baby after transplantation to receptive females [42].
Recently on 2011 Sato, et al. [43] reported first successful in vitro sperm production from immature germ cells. These scientists used organ culture and could produce healthy sperms that could fertilize the egg and led to pregnancy [43]. Most studDiscussion ies have been based on differentiation of spermatogonial stem cells into sperm. Nayernia, et al. [34] reported successful differentiation of embryonic stem cells to haploid mouse gamete [34]. However there is no report of human somatic cells ability to differentiate into haploid spermatid. Human granulosa cells are somatic cells that have stem cells characteristics. Oct-4 which its role in remaining self-renewing ability of stem cells and their pluripotency is known has expression in unfertilized oocytes [44]. According to the previous studies, cell surface markers CD29, CD90, CD44, CD105, CD117 and CD166 are expressed in GCs. These cells ability to differentiate into three types of cells including chondrocyte, osteoblast and neurons is also shown [8].
In the present study, mesenchymal stem cell markers analysis showed that CD44 which has a role in cell migration and cell homing and also CD105, CD106, CD146 and CD166 which all have the role in differentiation capacity of cells had high expression in the cells. Obtained results confirm previous findings. Since these cells show the properties of stem cells, their differentiation into sperm-like cells is likely. To evaluate the differentiation potential of GCs to sperm like cells markers of different steps of spermatogenesis were analyzed. Gpr125 protein known as spermatogonial cell marker was used as a first marker of differentiation. Its expression from the second week showed that GCs have entered the spermatogenesis pathway. Expression of VASA and DAZL proteins started from the second week and increased during the 2 other weeks. These proteins have the most expression before meiosis during spermatogenesis. These results indicate that differentiation could proceed until the meiosis stage of spermatogenesis and show the ability of GCs to differentiate into sperm like cells. Morphological adaptation of the produced cells with a normal sperm also confirmed obtained results [45].
Conclusion
Morphological analysis and expression of different spermatogenesis marker proteins indicates that, GCs can differentiate into sperm lineages and may exist new potentials in reproductive biology and clinical treatments.
Declaration of Interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgments
This study was conducted at the stem cell research center of ACECR (Academic center of Education, Culture and research). We wish to thank the staffs of this institute. We also thank Royan institute for their technical assistance.
- Smith AG (2001) Embryo-Derived Stem Cells: Of Mice and Men. Ann Rev Cell Develop Biol 17: 435-62.
- Fritsch MK, Singer DB (2008) Embryonic stem cell biology. Adv Pediatr 55: 43-77.
- Friedenstein AJ, Petrakova KV, Kurolesova Al, Frolova GP (1968) Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6: 230-47.
- In Anker PS, Scherjon SA, Kleijburg-van Dder Keur C, de Groot-Swings GM, Claas FH, et al. (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placena. Stem cells 22: 1338-45.
- Anderson RA, Sciorio R, Kennel H, Bayne RA, Thong KJ, et al. (2009) Cumulus gene expression as a predictor of human oocyte fertilization, embryo development and competence to establish a pregnancy. Reproduction 138: 629-37.
- Russel DL, Robker RL (2007) Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 13: 289-312.
- Rodgers RJ, Lavranos TC, van Wezel IL, Irving- Rodgers HF (1999) Development of the ovarian follicular epithelium. Mol Cell Endocrinol 151: 171-9.
- Kossowska- Tomaszczuk K, De Geyter C, De Geyter M, Martin I, Holzgreve W, et al. (2009) The multipotency of luteinizing granulosa cells collected from muture ovarian follicles. Stem Cells 27: 210-9.
- Russo V, Berardinelli P, Martelli A, Di Gioacinto O, Nardinocchi D, et al. (2006) Expression of telomerase transcriptase subunit (TERT) and telomere sizing in pig ovarian follicles. J. Histochem. Cytochem 54: 443-55.
- Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, et al. (2000) Cloned pigs Produced by nuclear transfer from adult somatic cells. Nature 407: 86-90.
- Park KW, Kuhholzer B, Lai LX, Machaty Z, Sun QY, et al. (2001) Development and expression of the green fluorescent protein in porcine embryos derived from nuclear transfer of transgenic granulosa-derived cells. Anim Reprod Sci 68: 111-20.
- Varras M, Griva T, Kalles V, Akrivis C, Paparisteidis N (2012) Markers of stem cells in human ovarian granulose cells: is there a clinical significance in ARST? J Ovarian Res 5: 36.
- Griswold MD (1998) The central role of Sertoli cells in spermatogenesis. Semin Cell Biol 9: 411-6.
- Amann RP, SS Howards (1980) Daily Spermatozoal Production and EpididymalSpermatozoal Reserves of the Human Male. J Urol 124: 211-5.
- Reijo R, RK Alagappan, P Patrizio, DC Page (1996) Severe Oligozoospermia Resulting from Deletions of Azoospermia Factor Gene on Y Chromosome. Lancet 347: 1290-3.
- Clermont Y (1963) The cycle of the seminiferou epithelium in man. Am J Anat 112: 35-51.
- Clermont Y (1966) Renewal of spermatogonia in man. Am J Anat 118: 509-24.
- Clermont Y (1972) Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 52: 198-236.
- Brinster RL (2002) Germline stem cell transplantation and transgenesis. Science 292: 2174-76.
- Brinster RL (2007) Male Germline stem cells: from mice to men. Science 316: 404-5.
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, et al. (2007) Generation of functional multipotentadult stem cells from GPR125+ germlineprogenitors. Nature 449: 346-50.
- He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M (2010) Isolation, characterization, and culture of human spermatogonia. Biol Report 82: 363-72.
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PT (2007) Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biol 7: 136.
- Yen PH (2004) Putative biological functions of the DAZ family. Int J Androl 27: 125-9.
- Kerr CL, Cheng L (2010) The dazzle in germ cell differentiation. J Mol Cell Biol 2: 26-9.
- Lin YM, Chen CW, Sun HS, Tsai SJ, Lin JS, et al. (2002) Presence of DAZL transcript and protein in mature human spermatozoa. FertilSteril 77: 626-9.
- Reijo RA, Dorfman DM, Slee R, Renshaw AA, Loughlin KR, et al. (2000) DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biol Reprod 63: 1490-6.
- Stefanidis K, Loutradis D, Koumbi L, Anastasiadou V, Dinopoulou V, et al. (2008) Deleted in Azoospermia-Like (DAZL) gene-expressing cells in human amniotic fluid: a new source for germ cells research. FertilSteril 90: 798-804.
- Nishi S, Hoshi N, Kasahara M, Ishibashi T, Fujimoto S (1999) Existence human DAZLA protein in the cytoplasm of human oocytes. Mol Hum Reprod 5: 495-7.
- Mc Neilly JR, Saunders PT, Taggart M, Cranfield M, Cooke HJ, et al. (2000) Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology 141: 4284-94.
- Gustafson EA, GM Wessel (2010) Vasa Genes: Emerging Roles in the Germ Line and in Multipotent Cells. Bioessays 32: 626-37.
- Yuan L, JG Liu, J Zhao, E Brundell, B Daneholt, et al. (2000) The Murine Scp3 Gene Is Required for Synaptonemal Complex Assembly, Chromosome Synapsis, and Male Fertility. Molecular Cell 5: 73-83.
- Schlegel PN (2009) Evaluation of male infertility. Minerva Ginecol 61: 261-83.
- Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, et al. (2006) In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell 11: 125-32.
- Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, et al. (2004) Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet 13: 727-39.
- Yao L, Yu X, Hui N, Liu S (2011) Application of iPS in assisted repro-ductive technology: sperm from somatic cells? Stem Cell Rev 7: 714-21.
- Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, et al. (2011) Complete meiosis from human induced pluripotent stem cells. Stem Cells 29: 1186-95.
- Maleki M, Ghanbarvand F, Behvarz MR, Ejtemaei M, Ghadirkhomi E (2014) Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int J Stem Cells 7: 118-26.
- Rassoulzadegan M, Paquis-Flucklinger V, Bertino B, Sage J, Jasin M, et al. (1993) Transmeiotic differentiation of male germ cells in culture. Cell 75: 997-1006.
- Hofmann MC, Hess RA, Goldberg E, Millán JL (1994) Immortalized germ cells undergo meiosis in vitro. Proc Natl Acad Sci U S A 91: 5533-7.
- Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, et al. (2002) Generation and in vitro differentiation of a spermatogonial cell line. Science 297: 392-5.
- Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, et al. (2002) Generation and in vitro differentiation of a spermatogonial cell line. Science 297: 392-5.
- Tesarik J, Bahceci M, Ozcan C, Greco E, Mendoza C (1999) Restoration of fertility by in-vitro spermatogenesis. Lancet 353: 555-6.
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. (2011) In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471: 504-7.
- Samardzija C, Quinn M, Findlay JK, Ahmed N (2012) Attributes of Oct4 in stem cell biology: perspectives on cancer stem cells of the ovary. J Ovarian Res 5: 37.
- Clermont Y (1966) Spermatogenesis in man. A study of the spermatogonial population. Fertil Steril 17: 705-21.

Figures at a glance