Combined Medical and Surgical Management of Unruptured Large Interstitial Ectopic pregnancy: Case Report and Literature Review
Received Date: April 07, 2020 Accepted Date: April 23, 2020 Published Date: April 25, 2020
doi: 10.17303/jwhg.2020.7.201
Citation: Shittu SA (2020) Combined Medical and Surgical Management of Unruptured Large Interstitial Ectopicpregnancy: Case Report and Literature Review. J Womens Health Gyn 7: 1-6.
Abstract
Background: True interstitial ectopic pregnancy (IEP) occurs in the intramural segment of the fallopian tube. It is a rare variant of ectopic pregnancy that carries a risk of life-threatening hemorrhage when it ruptures. Early diagnosis is the key to appropriate management.
Case Presentation: A 34-year old gravida 4 para 3 with 3 previous cesarean sections with a history of amenorrhoea of 13 weeks and mild lower abdominal pain was diagnosed with the large right IEP at diagnostic laparoscopy. The procedure was converted to laparotomy to avoid delay in converting laparoscopy to laparotomy due to adhesions in the face of torrential hemorrhage. Cornuostomy and ipsilateral total salpingectomy were done. Blood loss was 150mls. She was given intramuscular methotrexate postoperatively for deeply embedded trophoblastic tissues. She made an uneventful recovery and was discharged home on the 4th postoperative day.
Conclusion: There is no single gold standard treatment for interstitial ectopic pregnancy. Each case needs to be individualized and managed with approach and techniques that are safest, minimize blood loss, and best preserve fertility.
Introduction
Interstitial ectopic pregnancy IEP is defined as pregnancy implanted in the interstitial portion of the fallopian tube that is within the intramuscular wall of the uterus lateral to the round ligament. It is a rare variant (2-4%) of ectopic pregnancies that carries a risk of life-threatening intraperitoneal haemorrhage [1-4]. Hence, its mortality rate (seven times more) ranges from 2 to 2.5% compared to 0.14% for all other ruptured ectopic pregnancies. This is because most ruptures are reported later (12 -16 weeks) than in other tubal pregnancies because the myometrium is more distensible than the fallopian tube, but this common belief has been dispelled by more recent case series, reporting rupture at less than 12 weeks [1,2]. It is difficult to diagnose both clinically and sonographically [2].
Traditionally the treatment used to be laparotomy, hysterectomy, or cornual wedge resection. Significant progress has been made in the diagnosis and management of IEP based on widespread access to dedicated early pregnancy assessment units, high-resolution transvaginal ultrasound scan leading to earlier and more accurate diagnosis and so increasing the use of the more conservative method of treatment that has lower morbidity. These can be classified into expectant, medical, and surgical management [4-5].
We present a case of unruptured large right IEP that was successfully managed with combined conservative surgical and medical options of treatment.
Case Presentation
A 34-year-old gravida 4, Para 3 with 3 previous cesarean sections was referred from a private clinic on account of ultrasound confirmed missed miscarriage at 11 weeks. She had a history of amenorrhoea of 13 weeks and mild lower abdominal pain. No vaginal bleeding. Her vital signs were normal. Hemoglobin was 11.4g/l, and serum βHuman Chorionic Gonadotrophin (βhCG) was 55,904 iu/ml. Transvaginal ultrasound showed empty uterus and right adnexal ring-shaped highly vascular lesion measuring 4.3x3.5cm and a trace of fluid in the Pouch of Douglas suggesting ectopic pregnancy? corpus luteum cyst. Diagnostic laparoscopy was suggested because of the inadequacy of the ultrasound scan and a high index of suspicion of interstitial ectopic pregnancy.
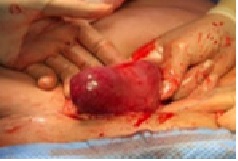
She consented to diagnostic laparoscopy which confirmed a large highly vascular unruptured right interstitial ectopic pregnancy measuring 4.5 x 4cm with impending rupture as the gestation was only covered at the top by a very thin serosal layer. In view of adhesions from the 3 previous abdominal surgeries, the procedure was converted to laparotomy to safely perform cornuostomy (with the removal of the fetus and other products of conception) and ipsilateral salpingectomy. The estimated blood loss was 150mls. She had postoperative single-dose intramuscular methotrexate (50mg/m2) to treat deeply embedded trophoblastic tissue. She made good recovery and was discharged home on the on the 4th postoperative day. Serum BhCG was checked weekly on outpatient basis till it was < 5iu and histopathology testing confirmed products of conception and right fallopian tube.
Discussion
Diagnosis of tubal ectopic pregnancy has become easy with the advent of transvaginal ultrasound and sensitive serumβhCGassay combined with a high index of suspicion. However, IEP remains the most difficult ectopic pregnancy to diagnose before surgery [6]. The patient was misdiagnosed as a missed miscarriage in a private hospital and diagnosis of IEP was only made at diagnostic laparoscopy confirming the fact that interstitial ectopic pregnancy remains one of the most difficult gestations to detect. Delayed diagnosis is the main factor contributing to the high maternal mortality rate in comparison to that of tubal ectopic pregnancies [5-6].
The diagnostic criteria for interstitial ectopic pregnancy are:
1. Empty uterine cavity.
2. Gestational sac located laterally in the interstitial (intramural) part of the tube and surrounded by less than 5 mm of myometrium in all imaging planes.
3. A chorionic sac separated 1cm from the most lateral edge of the uterine cavity
4. The ‘interstitial line sign’, - a thin echogenic line extending from the central uterine cavity echo to the periphery of the interstitial gestational sac [6-8].
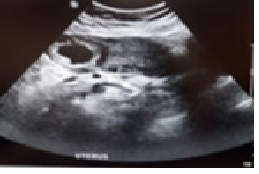
These parameters have 88 – 93% specificity but the sensitivity was only 40% for the diagnosis of IEP. The demonstration of the interstitial line sign has a sensitivity of 80% and a specificity of 98% [6]. Eccentrically located gestational sac surrounded by an asymmetric myometrial mantle and a separate empty uterine cavity with endometrial echoes were the commonest ultrasonographic findings of interstitial gestation [7-9] as shown in (Figure 1) and demonstrated in (Figure 2).
Three-dimensional ultrasonography and supplementation with MRI can be helpful in non-urgent cases. The diagnosis may be confirmed on MRI by the presence of an uninterrupted junctional zone separating the gestational sac from the endometrium [9].
The differential diagnoses include angular pregnancy, isthmic tubal pregnancy, and cornual ectopic pregnancy.
Angular pregnancy occurs when an embryo is implanted medial to the utero-tubal junction in the lateral angle of the uterine cavity very near the internal ostium of the fallopian tube. The pregnancy is medial to the round ligament. The clinical course varies widely; it can progress to the second trimester or even to term but is associated with high rates of spontaneous abortion, uterine rupture, and placenta accreta. Miscarriage and rupture can occur in as many as 38.5% and 23.5% of all angular pregnancies [10-12].
In Isthmic pregnancy, especially stump pregnancy interstitial line sign is absent, and the gestational sac appears in the adnexal region without the surrounding myometrium on pelvic ultrasound [4,8].
On the other hand, a cornual ectopic pregnancy is one in which the embryo implants within the endometrium of the horn of a unicornuate uterus or the lateral half of a bicornuate or septate uterus. The risk of rupture is dependent on the expansive capability of the uterus at the implantation site. Cornual ectopic pregnancy is often used synonymously with interstitial ectopic pregnancy, however, despite the controversies surrounding their definitions, these conditions follow different clinical courses. MRI may also be helpful in differentiating an interstitial or cornual ectopic pregnancy from an intrauterine pregnancy in an anomalous uterus, such as a bicornuate uterus.
Predisposing factors are like those for any ectopic pregnancy: these include pelvic inflammatory disease from sexually transmitted disease, previous ectopic pregnancy, pelvic surgery, tumors, uterine anomalies, and assisted reproductive technology. Ipsilateral (or bilateral salpingectomy) is the only risk factor that exists exclusively for interstitial ectopic pregnancy [12].
Common symptoms of IEP are amenorrhoea, abdominal pain, and vaginal bleeding. Some may be asymptomatic or have their IEP diagnosed on routine early pregnancy scan. Also, some patients may present with signs of acute abdomen with rupture and hemodynamic instability (shock) requiring immediate laparotomy before a non-invasive diagnosis may be made [13-15].
The overall decision regarding management options depends upon clinical presentation, size of the IEP, presence of fetal cardiac activity, the serum βHCG level, the expertise of the specialist, and resources available [16]. The treatment can be expectant, medical, or surgical. The current trend is to offer conservative treatment in the form of medical management (systemic methotrexate) or laparoscopic approach (without or with hysteroscopy and ultrasound guidance) [16,17].
Expectant management of interstitial ectopic pregnancy has not been widely used because of concern with catastrophic bleeding or because of uncertainty about the diagnosis. It is a feasible option in well-selected asymptomatic women with non-viable interstitial pregnancy and declining serum beta HCG. It should be abandoned if there are clinical features of rupture or impending rupture (increasing serum bHCG, pain, and haemoperitoneum) [16-18].
Adequate counseling and follow up that can be very prolonged are essential and the patient must be aware of the risk of rupture and the potential need for emergency surgery. Her choice must be considered. Poon et al demonstrated a success rate of 89.5% and no case of a rupture in their cohort of patients managed expectantly with a length of follow up ranging from 7 to 141 days [15]. The benefit of expectant management is that it is non-invasive, and it avoids the potential side effects of methotrexate or surgery [16].
Medical management with methotrexate has been used as first-line treatment in appropriate cases using strict inclusion criteria (early gestation, diameter < 4 cm, serum βhCG of < 10,000 IU/l and no evidence of rupture) and treatment was shown to be successful in cases that satisfy these criteria and in women who are able to be monitored for a quite long time after treatment and treated further if required [5,6,15,16].
Jermy K, et al. concluded that systemic methotrexate is a safe and highly effective treatment for IP with the advantage that surgery is avoided in the majority of the patient using a single dose (50mg/m2), intramuscular methotrexate was administered on day 0. A second dose of methotrexate was given if the beta-hCG levels had not fallen by 15% between days four and seven. Weekly follow up continued until the serum beta-hCG< 5 IU. The success rate of 95% is documented [5].
Though systemic injection of methotrexate (MTX) is the most extensively studied medical regimen, local injection of methotrexate (1mg/kg) has the benefit lower dose and hence lower risk of side effects such as transient peripheral neuropathy, severe constipation, and deterioration in liver function. It can be done under laparoscopic guidance or with ultrasound via the transvaginal route using a fine spinal needle. It is more invasive and operator dependent. Rupture can still occur despite declining HCG levels [5,10,17]. Our patient was not managed medically alone in view of the fact that she was having lower abdominal pain, size of the interstitial ectopic gestation and the fact that laparoscopy showed she was close to rupture (judging by the thin wall of the gestation) and bleeding can be catastrophic if this should happen.
Surgery offers a definitive treatment for IEP and is indicated when patient refuses, fails, or is unsuitable for medical treatment, in the presence of significant symptoms, in ruptured cases, heterotopic pregnancies, or in large interstitial ectopic and with the presence of a heartbeat [6]. Historically, laparotomy, cornual wedge resection or hysterectomy used to be done. These days, wedge resection should only be done in cases of ruptured IEP with troublesome bleeding [18].
Even though laparoscopy is the preferred approach because of less postoperative pain, reduced hospital stay and earlier return to normal function and work and better cosmetic advantage, laparotomy remains a suitable alternative that will always produce a safe outcome. In our patient, laparotomy was used due to large size (>4 cm) of the highly vascular ectopic pregnancy with a very thin wall suggesting impending rupture and because the rapid conversion of laparoscopy to laparotomy may be hindered by adhesions from 3 previous cesarean sections in the face of torrential bleeding. The ipsilateral fallopian tube was excised in this case to avoid recurrence of ectopic gestation on the right side.
The current trend is to use conservative surgical alternatives to cornual wedge resection (cornuostomy or cornuectomy) in an attempt to increase future fertility and decrease the risk of uterine rupture during a subsequent pregnancy. These conservative surgical treatments successfully used a combination of hysteroscopic, laparoscopic, and ultrasound-guided transcervical evacuation of IEP [18-19].
Use of manual vacuum aspiration under laparoscopic guidance and novel use of hysteroscopic urologic stone retrieval forceps in the transvaginal removal of persistent products of conception after systemic methotrexate were reported by Grindler N, et al. [10].
Intraoperative bleeding is a major problem for laparoscopic management because of the rich supply of blood vessels at the location of the IEP. Data from safe use of conservative surgical techniques that preserve fertility and conserve blood loss with the use of electro-diathermy, vasopressin, intra, and extra-corporeal suturing, fibrin glue, and endoloops appear reassuring [14]. Successful laparoscopic management of IEP using the automatic stapler to simultaneously excise and stitch the uterine cornua has also been reported [20].
One of the key challenges after laparoscopic surgery is the persistent trophoblastic activity even after cornual resection which may be due to increased myometrial invasion in some IEP. This has been treated successfully with methotrexate as we did for our patient [3,6,18,19].
The possibility of recurrent IEP and uterine rupture during labour in subsequent pregnancies and as such they need for an early antenatal ultrasound and cesarean delivery should be discussed with women undergoing treatment for IEP [11].
Conclusion
Though case series report high rates of success with medical management as the first line of treatment., this case reflects the fact that there is no single gold standard treatment for IEP. Each case needs to be individualized and managed using approach and techniques that are safest, minimize blood loss, and best preserve fertility [17,18,19].
- Khoiwal K, Kumari O, Gaurav A, Kapur D, and Chaturvedi J (2019) Interstitial Tubal Ectopic Pregnancy: Case Report and Review of the Literature. Journal of Gynecologic Surgery 35: 5.
- Faraj R, Steel M (2007) Review Management of cornual (interstitial) pregnancy. TOG 9: 249-255.
- MacRae R, Olowu, Rizzuto MI, Odejinmi F (2009) Diagnosis, and laparoscopic management of 11 consecutive cases of cornual ectopic pregnancy. Arch Gynecol Obstet 280: 59-64.
- T E Ackerman, CS Levi, S M Dashefsky, S C Holt and DJ Lindsay (1993) “Interstitial line: Sonographic finding in interstitial (cornual) ectopic pregnancy,” Radiology 189: 83–87.
- K Jermy, J Thomas, A Doo and T Bourne (2004) “The conservative management of interstitial pregnancy,” BJOG: An International Journal of Obstetrics & Gynaecology 111: 1283–1288.
- Yassin AS, Taha MS (2017) Interstitial Ectopic Pregnancy, Diagnosis and Management: A Case Report and Literature Review. Ann Clin Case Rep 2: 1352.
- GF Grimbizis, T Tsalikis, T Mikos, et al. (2004) "Case report: Laparoscopic treatment of a ruptured interstitial pregnancy," Reproductive Bio Medicine Online 9: 447–451.
- Linda Y. Kao, et al. (2014) Beyond Ultrasound: CT and MRI of Ectopic Pregnancy. American Journal of Roentgenology 202: 904-911.
- T Tulandi and D Al-Jaroudi (2004) “Interstitial pregnancy: Results generated from the society of reproductive surgeons registry,” Obstetrics & Gynecology 103: 47–50.
- Natalia M Grindler, June Ng, Kristina Tocce and Ruben Alvero (2016) Consideration for management of interstitial ectopic pregnancies: two case reports. Journal of Medical Case Reports 10: 106.
- AM Abbas, FM Fawzy, MN Ali, and MK Ali (2016) “An unusual case of uterine rupture at 39 weeks of gestation after laparoscopic cornual resection: A case report,” Middle East Fertility Society Journal 21: 196–198.
- S Lau and TTulandi (1999) “Conservative medical and surgical management of interstitial ectopic pregnancy,” Fertility and Sterility 72: 207–215.
- HS Moon, SG Kim, GS Park, JK Choi, JS Koo and BS Joo (2010) “Efficacy of bleeding control using a large amount of highly diluted vasopressin in laparoscopic treatment for interstitial pregnancy,” American Journal of Obstetrics & Gynaecology 203: 30–e6.
- HS Moon, YJ Choi, YH Park, and SG Kim (2000) “New simple endoscopic operations for interstitial pregnancies,” American Journal of Obstetrics & Gynaecology 182: 114–121.
- Poon LCY, et al. (2014) How feasible is the expectant management of interstitial ectopic pregnancy? Ultrasound Obstet Gynecol 43: 317 – 325.
- Jermy K, Thomas J, Doo A, Bourne T (2004) The conservative management of interstitial pregnancy. BJOG 111: 1283.
- Cassik P, et al. (2005) Factors influencing the success of conservative treatment of Interstitial ectopic pregnancy. Ultrasound Obstet Gynecol 26: 279-282.
- R MacRae, O Olowu, MI Rizzuto & F Odejinm (2009) Diagnosis and laparoscopic management of 11 consecutive cases of cornual ectopic pregnancy. Archives of Gynaecology and Obstetrics 280: 59-64.
- Woojin Chong, Nicolae Tudorica, Erika Banks (2017) Surgical Expression of an Un-Ruptured 12-Week Interstitial Ectopic Pregnancy. Mathews Journal of Gynaecology & Obstetrics 2: 1.
- Sergent F, Le Cornec JB, Meilhaud MF, Marpeau L (2003) Laparoscopic cornual excision with an automatic stapler for ruptured interstitial pregnancies. J Gynecol Obstet Biol Reprod 32: 426.

Figures at a glance