Solitary Fibrous Tumor of Nasal Cavity with Intracranial Extension
Received Date: April 17, 2020 Accepted Date: April 30, 2020Published Date: May 02, 2020
doi: 10.17303/jspcr.2020.2.101
Citation:M’jahad N (2020) Solitary Fibrous Tumor of Nasal Cavity with Intracranial Extension. J Surg Proce Case Rep 1:1-7.
Abstract
Introduction: The solitary fibrous tumor is a mesenchymal tumor. Relatively rare in the head and neck. The sino-nasal localization is rare, as is the intracranial Extension. The diagnosis is histological, the tumor is made of a dense proliferation of fusiform cells. The immune-histochemical study of solitary fibrous tumor classically shows diffuse expression of vimentin and CD34, intense and diffuse nuclear staining of STAT-6 is highly characteristic of this tumor. The treatment is the complete surgical resection but can be challenging, because of the tumor’s firm consistency and adhesion to neighboring structures. Requiring in this kind of localization multidisciplinary collaboration between ENT, neurosurgeon, and radiotherapist. Close control is necessary because of the risk of recurrence or metastasis.
Case report: The eighty-year-old man presented with a mass of the right nasal cavity. The patient underwent cranio-facial computed tomography (CT) and magnetic resonance (MR) imaging which showed a locally advanced right nasal cavity tumor process with intracranial extension. An endoscope excision of the tumor was performed with reconstruction. Histopathological analysis of the specimen was consistent with a malignant solitary fibrous tumor. The patient progressed well postoperatively. The patient was then referred to radiotherapy for additional treatment after the decision by the Multidisciplinary consultation meeting.
Conclusion: In the light of a new and very demonstrative observation and literature data, we wanted to review the clinical, endoscopic and radiological, histological, immunohistochemical profile of this particular type of tumor as well as the therapeutic aspect. Keywords: solitary fibrous tumor; intracranial extension; computed tomography (CT); magnetic resonance (MR) imaging; surgery
Introduction
Described for the first time in the pleura by Klemperer and Rabin in 1931 [1]. Solitary fibrous tumors are rare mesenchymal tumors, the first reported case of the solitary fibrous tumor (SFT) in the region of the head and neck was in 1991 [2]. Through a nasal localization with endocranial extension and a review of the literature, the authors propose to recall the epidemiological, clinical, endoscopic, radiological, histological, and therapeutic particularities of this rare localization of solitary fibrous tumors.
Case report
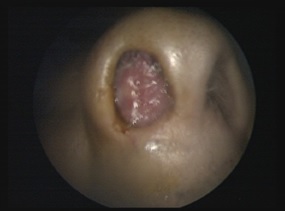
H, B aged 80 years with no notable pathological history who complained of a right nasal obstruction associated with epistaxis of low abundance with repetition, going back to 8 months before his consultation, without other associated signs, in particular, no facial pain, and visual disorders due to compression of the orbit, or signs neurological. The rhinological examination objectified the presence of a budding process completely obstructing the right nasal cavity not bleeding on contact with deviation from the nasal septum towards the left side (Figure 1). The eye exam is normal. Oral and neurological exams, including cranial pair exams, are normal. The lymph nodes are all free.
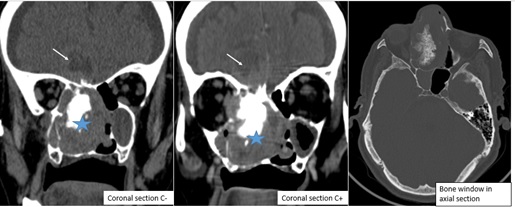
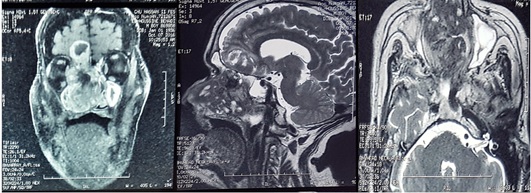
The patient initially underwent cranio-facial computed tomography (CT) showed a necrotic tumor process of the right nasal cavity with the presence of significant calcifications, with extension towards the anterior and posterior ethmoid cells homolateral and the right palate, bone lysis in the horizontal portion of the frontal bone associated with a bulky frontal process of 21 mm, filling of the frontal and right sphenoid sinuses, total filling of the right maxillary sinus and partial of the left maxillary sinus with an endocranial extension( figure 2). A complementary cranio-facial MRI was performed objectifying a process of the right nasal cavity in heterogeneous hypoT1 and heterogeneous hyper T2, heterogeneous enhancement with the injection intravenous of gadolinium, delimiting zones of necrosis and containing calcifications, the mass appears to be pedicled superiorly to the dura of the anterior cranial fossa with extension to the right basifrontal intracerebral level; extending into the nasopharynx, it infiltrates the homolateral sphenoid sinus and the inner wall of the right orbit with lysis of the nasal septum (figure 3).
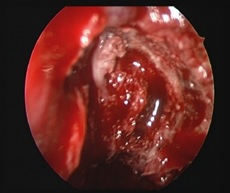
A biopsy under local anesthesia was performed and the histological study concluded at histiocytofibroma. After consultation with anesthesia and patient consent; The patient was operated in collaboration between the Otorhinolaryngologists team (ENT) and the neurosurgery team, by endonasal endoscopic, using neuronavigation, having benefited from a total excision of the tissue process of the nasal cavity in one piece with maxillectomy, sphenoidectomy, and ethmoidectomy (figure 4). After the opening of the dura mater, The tumor was poorly vascularized, whitish, encapsulated, presenting a plan of cleavage with the cerebral parenchyma, and excision by fragmentation of the intracranial tumor was carried out the excision was difficult with the risk of hemorrhage and damage to the adjacent structure due to the extension of the tumor. He was transfused with 2 red blood cells intraoperatively. The macroscopic pathological examination revealed a mass of 5 cm * 4.5 cm of a brownish color and firm consistency
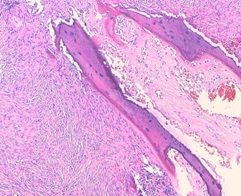
The histological examination revealed high cellular proliferation made of bundles and storiform structures. Tumor cells were fusiform with moderate cytonuclear atypia. There were 6mitosis /10high power fields. Hemangiopericytoma-like (figure 5) vessels were noted. This proliferation infiltrates the bone tissue and came in contact with the cerebral parenchyma. An immunohistochemical study was carried out: the tumor cells express the anti CD34 antibody, the anti bcl2 antibody, and a clear expression of STAT6. The proliferation index evaluated by the Ki67 is estimated at 20%. The diagnosis of the malignant solitary fibrous tumor was retained (high cell density, the cytonuclear atypia, and the high mitotic activity). The patient received postoperative radiotherapy. The patient progressed well postoperatively, in particular no fever or signs of intracranial hypertension, with satisfactory radiological control (Figure 6): the presence of minimal pneumocephalus with the completely unobstructed nasal cavity, without recurrence or metastasis, with a follow-up of 36 months.
Discussion
A solitary fibrous tumor (SFT), is a fibroblastic mesenchymal tumor that was first described by Klemperer and Rabin in 1992 [1]. SFTs are rare entities accounting for < 2% of all soft tissue sarcomas [3]. Although SFTs are commonly found in the pleura, according to the literature, 50 - 70% of these tumors are extrapleural [4, 5].
The exact prevalence of primary head and neck SFTs is difficult to estimate, as most published cases were reported in very small series or combined with SFTs from other anatomical regions [6].
In the head and neck, the most common site affected is the oral cavity, but reports have documented cases in the orbit, nose and paranasal sinuses, nasopharynx, and parapharyngeal space, larynx, major salivary glands, and thyroid. Some cases exhibit an extension into the anterior cranial fossa [7, 8,9]. In general, SFTs may become manifest at any age, and there is no specific peak of age prevalence; with an equal distribution between the sexe [6,10].
The clinical presentation in the nose and paranasal sinuses is that of progressive nasal obstruction and / or secondary symptoms of obstruction or epistaxis. Depending on the intensity of the extension to neighboring structures, there may be visual signs, neurological signs by damage to the cranial or cerebral pairs. There is a firm, lobulated, and well-encapsulated mass. Medical imaging is based primarily on CT and MRI. Their results are not specific. It allows identification of tumors, the local extent, and invasion of adjacent structures, which is useful in guiding surgery.
The CT scan shows a very limited, heterogeneous, or isodense homogeneous mass, with contrast enhancement after the injection of contrast product [11,12]. MRI is the examination of choice for diagnostic orientation. The solitary fibrous tumor appears iso-intense compared to the cerebral gray matter in T1 sequence, with a homogeneous or heterogeneous enhancement with the injection intravenous of gadolinium. In the T2 sequence, the tumor is hypo-intense. The central part of the tumor is often hypo intense in T1 and T2. The distinctive radiological characteristics of the solitary fibrous tumor are the hypo-intense signal in T2 with heterogeneous intra-lesional signals [13-16].
MRI, therefore, seems to be the most appropriate imaging modality to reduce the differential diagnosis during preoperative planning [16, 17]. Based on these symptoms and appropriate radiological imaging, the clinical differential diagnosis includes benign anterior cranial lesions such as meningiomas, malignant lesions such as fibrosarcomas, hemangiopericytomas, nasopharyngeal carcinomas, or esthesioneuroblastomas [16,18].
The biopsy is made easy with endonasal endoscopy. The SFT diagnosis is supported by a characteristic immunohistochemical profile [19,20]; Macroscopically, the tumor is well limited and often encapsulated and translucent. The size varies generally between 1 cm and 25 cm [21]. At the cut, the surface appears fasciculate and lobulated. Its color is often greyish, sometimes pink-white [21]. Histologically, the tumor is made of a dense proliferation of fusiform cells, dispersed orderly, and supported by a frame of collagen fibers of variable abundance. It is characterized by branching hemangiopericytoma- like vessels. The main signs of malignancy are represented by a high mitotic index (greater than four mitoses per ten fields at high magnification), a high cell density, necrotico- hemorrhagic changes, marked nuclear pleomorphism and vascular invasion [22 -25]. The immune- histochemical study of SFT classically shows diffuse expression of vimentin and CD34, variable expression of CD99 and bcl-protein and negativity of the epithelial marker (cytokeratin, EMA) and of the protein S100 [26]. Intense and diffuse nuclear staining of STAT-6 is highly characteristic of SFT, seen in more than 90%of the cases [27]. Some factors of poor prognosis have been reported as the loss of expression of the CD34 antigen, the high expression of Ki67 (MiB1), and of the P5protein [28].
Microscopically, there are several differential diagnoses such as fibrous histiocytoma, fibromatosis, fibrosarcoma, and hemangiopericytoma. Immuno- histochemical study helps in distinguishing these entities [21]. The treatment of reference is complete surgical resection. Incomplete resection is a pejorative factor of ulterior evolution [29,30]. Endoscopic endonasal surgery is ideal for resection of benign encapsulated lesions; and if the surgeon has experience in advanced endoscopic techniques for potentially addressing endoscopic bleeding quickly
Studies have also demonstrated that preoperative embolization may be employed prior to surgical resection for highly vascular tumors [31, 32]. Immunotherapy, for example with Interferon, may also be effective [33].
The role of other treatments is not yet well codified. Radiotherapy could also be indicated after surgical resection, in cases of histological signs of malignancy, especially if this resection is incomplete [30]. Xue and al [34] described a case of non-recurrent malignant SFT of the nasal and paranasal areas and found that the combination of cytoreductive surgery with intensity-modulated radiation and stereotactic body radiation therapies have a good result.
An in vitro study, testing the sensitivity of a solitary fibrous tumor with a mitotic index of 7/50 HPF with chemotherapy, the tumor was sensitive to 5-FU, adramycin, mitomycin-c, and doctaxel [35]. Chemotherapy may be interesting in inoperable malignant forms, in neo-adjuvant, in large tumors, or in adjuvant, in cases of incomplete resection [30,35].
There has only been one previous report of an SFT recurrence in the upper respiratory tract. Factors that predispose to local recurrence in non- head, and neck SFTs are a tumor diameter larger than 10 cm, the presence of a malignant component in the histological findings, and microscopically positive surgical margins [36]. In contrast to tumors found in other anatomical locations, tumors in the head and neck region present a unique anatomical challenge, due to their close relationship to neurovascular structures and distinct anatomical compartments. Patients who undergo complete surgical resection and do not have any malignant components can expect an excellent outcome with 100% survival at an average follow-up of 1.9 years. A high proliferation index at the time of diagnosis appears to be another risk factor for aggressive tumor behavior. Although it is generally benign, the malignant variants have been identified. Metastases have been reported only in sites other than the head and neck [37]. In their review, Cox and al. Identified 10 cases in which there were atypical or malignant features, out of the 142 cases of SFT in the head and neck reported to date [38].
Solitary fibrous tumor/hemangiopericytoma (SFT/ HPC) is a new combined entity for which a soft-tissue–type grading system, ranging from grades I to III, has been introduced in 2016 WHO classification of tumors of the CNS(central nervous system)[10].In their review, Kyoung Su Sung and al. Identified
The SFT/HPC grade I group showed a relatively benign course compared with those of the other groups. The grade III group presented a course with a more aggressive nature than that of the grade II group. In the grade II group, the extent of resection and adjuvant RT was significantly associated with longer PFS (progression-free survival) [39]. Close control is necessary because of the risk of recurrence or metastasis
Conclusion
The solitary fibrous tumor of the nasal cavity with endocranial extension is rare. Its diagnosis is histological. This location poses a great problem of care. The treatment of choice is the complete surgical resection, while radiotherapy and conventional chemotherapeutic agents have only shown limited efficacy, a better understanding of the molecular events underlying tumorigenesis could allow the development of new targeted treatments. Surgical excision is a very important factor in the success of treatment and the decrease in the frequency of recurrences. Currently, follow-up is recommended due to unpredictable behavior with potential for recurrence and significant malignancy
Conflicts of interest: The authors declare that they have no conflicts of interest.
Author contributions: All authors have contributed to the diagnostic and therapeutic management of patients and to the writing of this work. All the authors contributed to the conduct of this work. All authors also declare that they have read and approved the final version of the manuscript.
- Klemperer P, Rabin CB (1931) Primary neoplasm of the pleura: a report of five cases. Arch Pathol. 11: 385-412.
- Zukerberg LR, Rosenberg AE, Randolph G, Pilch AE (1991) Solitary fibrous tumor of the nasal cavity and paranasal sinuses. Am J Surg Pathol 15: 126–130
- Gold JS, Antonescu CR, Hajdu C, Ferrone CR, et al. (2002) Clinicopathologic correlates of solitary fibrous tumors. Cancer 94: 1057-1068
- van Houdt WJ, Westerveld CM, Vrijenhoek JE, van Gorp J, et al. (2013) Prognosis of solitary fibrous tumors: A multicenter study. Ann Surg Oncol. 20: 4090-4095.
- Weige, DE-CAI YU, Gang Chen, and YI-TAO Ding (2016) Clinical analysis of 47 cases of solitary fibrous tumor; oncology letters 12: 2475-2480.
- Yu-Chien Kao, Po-Chun Lin, Chien-Feng Li, et al. (2016) Clinicopathological and genetic heterogeneity of the head and neck solitary fibrous tumors: a comparative histological, immunohistochemical and molecular study of 36 cases; Histopathology 68: 492–501.
- Hicks DL, Moe KS (2004) Nasal solitary fibrous tumor arising from the anterior cranial fossa. Skull Base 14: 203–207.
- Kim TA, Brunberg JA, Pearson JP, Ross DA (1996) Solitaryfibrous tumor of the paranasal sinuses: CT and MR appearance.AJNR Am J Neuroradiol 17: 1767–1772.
- Chikh K, Fergoug I, Soltani H, Ghouadni M, & Mehadji M (2014) Tumeur fibreuse solitaire avec extension endocrânienne. Annales Françaises d’Oto-Rhino-Laryngologie et de Pathologie Cervico-Faciale 131: A156–A157.
- Zeng L, Wang Y, Wang Y, Han L, Niu H, Zhang M, et al. (2017) Analyses of prognosis-related factors of intracranial solitary fibrous tumors and hemangiopericytomas help understand the relationship between the two sorts of tumors.J Neurooncol. 131: 153-161.
- Chis O and Albu S (2014) Giant solitary fibrous tumor of the parotid gland. Case Rep Med 950712, 2014.
- Li XM, Reng J, Zhou P, Cao Y, Cheng ZZ, et al. (2014) Solitary fibrous tumors in abdomen and pelvis: Imaging characteristics and radiologic-pathologic correlation. World J Gastroenterol 20: 5066-5073.
- Hyun Jeong Kim, MD Ho Kyu Lee (2005) MD 21. MR Imaging of Solitary Fibrous Tumors in the Head and Neck Hyun Jeong Kim, MD Ho Kyu Lee, MD 21. Korean J Radiol 6: 136-142.
- Ginat DT, Bokhari A, Bhatt S, Dogra V (2011) Imaging features of solitary fibrous tumors. AJR Am J Roentgenol 196: 487–495.
- Liu Y, Tao X, Shi H, Li K (2014) MRI findings of solitary fibrous tumours in the head and neck region. Dentomaxillofac Radiol 43: 20130415.
- C Dauleac, A Vasiljevic, M Berhouma (2020) Comment différencier l'hémangiopéricytome de la moelle épinière de la tumeur médullaire commune? Neurochirurgie. 66: 53-55.
- Julian Kunzel, Michael Hainz, Thomas Ziebart1 (2015) Head and neck solitary fibrous tumors: a rare and challenging entity. Eur Arch Otorhinolaryngol Springer-Verlag Berlin Heidelberg.
- Eloy PH, Nollevaux MC, Watelet JB, Van Damme JP, Collet ST, Bertrand B (2006) Endonasal endoscopic resection of an ethmoidal solitary fibrous tumor Eur Arch Otorhinolaryngol. 263: 833–837
- Hunt I, Ewanowich C, Reid A, Stewart K, Bédard EL, and Valji A (2010) Managing a solitary fibrous tumor of the diaphragm from above and below. ANZ J Surg 80: 370-371.
- Kita Y (2012) Pleural solitary fibrous tumor from the diaphragm, being suspected of liver invasion; report of a case. Kyobu Geka 65: 338-340
- JF Graadt, PC W Hogendoorn (2004) Solitary fibrous tumor: the emerging clinicopathologic spectrum of an entity and its differential diagnosis. Current Diagnostic Pathology 10: 229-235.
- Cardillo G, Facciolo F, Cavazzana A, Capece G, Gasparri R, Martelli M (2000) Localized (solitary) fibrous tumors of the pleura: an analysis of 55 patients. Ann Thorac Surg 70: 1808-1812.
- Léna H, Desrues B, Caullet-Maugendre S, Le Coz A, Huet H, Delaval P (1995) Le fibrome pleural : apport de l’immunohistochimie. Rev Mal Respir 12: 383-385.
- Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF (1997) Solitary fibrous tumor: a cytologic- histologic study with clinical, radiologic, and immunohistochemical correlations.Cancer 81: 116-121.
- England DM, Hochholzer L, Mc Carthy M (1989) Localized benign and malignant fibrous tumors of the pleura. Am J Sur Pathol 13: 640-658.
- Leroy X, Copin MC, Petit S, Moukassa D, Gosselin B (2001) Tumeur fibreuse solitaire pleuralemaligne avec expression focale de cytokératine. Ann Pathol 21: 153-156.
- Bita Geramizadeh, Mahsa Marzban, and Andrew Churg (2016) Role of Immunohistochemistry in the Diagnosis of Solitary Fibrous Tumor, a Review, Iran J Patholv.11: Summer PMC 507945.
- Jougon J, Minniti A, Bégueret H, Dromer C,Delcambre F, Velly JF, et al. (2002) Tumeur fibreuse solitaire de la plèvre. Rev Pneumol Clin 58: 35-38.
- Gengler C and Guillou L (2006) Solitary fibrous tumour and haemangiopericytoma: Evolution of a concept. Histopathology 48: 63-74.
- Liu H, Yang A, Chen N, Yang J, Qiu X, Zhang J (2013) Hemangiopericytomas in the spine: clinical features, classification, treatment, and long-term follow-up in 26 patients. Neurosurgery 72:16–24 discussion 24.
- Zerón-Medina J, Rodríguez-Covarrubias F, GarcíaMora A, Guerrero - Hernandez M, et al. (2011) Solitary fibrous tumor of the pelvis treated with preoperative embolization and pelvic exenteration. Am Surg 77: 112-113.
- Botchu R, Khan AN, Adair W, Elabassy M (2011) Solitary fibrous tumor made resectable after successful endovascular embolization. J Gastrointest Cancer 42: 287-291.
- Cuello J and Brugés R (2013) Malignant solitary fibrous tumor of the kidney: Report of the first case managed with interferon. CaseRep Oncol Med 564980.
- Xue Y, Chai G, Xiao F, Wang N, Mu Y, Wang Y, and Shi M (2014) Post-operative radiotherapy for the treatment of malignant solitary fibrous tumor of the nasal and paranasal area. Jpn J Clin Oncol 44: 926-931.
- Voshimasu T, Oura S, Hirai I, Kokawa Y, Nishida M, Sasaki R, et al. (2005) Retroperitoneal solitary fibrous tumor: about two observations. Prog Urol 15: 1128-1133.
- Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, et al. (2002) Clinicopathologic correlates of solitary fibrous tumors. Cancer 94: 1057–1068.
- Ito H, Fukuda M, Imamura Y, Fuse H (2008) A malignant solitaryfibrous tumor in the retroperitoneum. Int J Clin Oncol.13 :173–175
- Cox DP, Daniels T, Jordan RC (2010) Solitaryfibrous tumor of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 110: 79–84
- Kyoung Su Sung, MD, Ju Hyung Moon, MD, Eui Hyun Kim (2019) Solitary fibrous tumor/ hemangiopericytoma: treatment results based on 2016 WHO classification. J Neurosurg 130: 418–425


Figures at a glance