On the Origin of The Apparent Volume of Distribution and Its Significance in Solvent Extraction Methods
Received Date: January 12, 2023 Accepted Date: February 03, 2023 Published Date: February 06, 2023
doi: 10.17303/jocs.2023.1.101
Citation: Michalakis Savva (2023) On the Origin of The Apparent Volume of Distribution and Its Significance in Solvent Extraction Methods. J Org Chem Chem Sci 1: 1-7.
Abstract
The apparent volume of distribution was defined from solute distribution experiments as the phase volume required to occupy the total amount of solute in a system at the measured phase solute concentration under conditions of constant phase and system volumes, at equilibrium. This new property can increase the understanding of solute phase distribution, simplify calculations and in certain cases it can even supersede the use of the partition coefficient in solvent extraction methods.
Keywords:Apparent Volume of Distribution; Partition Coefficient; Closed System; Solvent Extraction
Introduction
During our study of compartment models, we stumbled upon a property called the apparent volume of distribution (Vd). The term apparent volume of distribution was used for the first time by Alexander Winkler and Paul Smith in 1938. In their study of electrolytes they were occasionally measuring distribution volume of potassium in dogs larger than the total body water [1]. There are hundreds of “definitions” for the apparent volume of distribution in the literature. Few examples are included herein: “The volume of distribution (also known as apparent volume of distribution, literally, volume of dilution) is the theoretical volume that would be necessary to contain the total amount of an administered drug at the same concentration that it is observed in the blood plasma. In other words, it is the ratio of amount of drug in a body (dose) to concentration of the drug that is measured in blood, plasma, and un-bound in interstitial fluid.”; “The apparent volume of distribution (Vz) of a drug is not literally a volume. It should not be regarded as a particular physiological space within the body. It is somewhat misleadingly described as the volume of body water which would be required to contain the amount of drug in the body if it were uniformly present in the same concentration in which it is in the plasma or blood. However, all regions in the body which contain drug will not have equal concentrations, so any volume calculated utilizing the drug concentration in plasma can only be an apparent volume. It is most prudent to avoid all analogies to volumes and define (Vz) as a proportionality factor which, when multiplied by the concentration of drug in the plasma, yields the amount of drug present in the body.”; “The volume of distribution represents a volume that must be considered in estimating the amount of drug in the body from the concentration of drug found in the sampling compartment. The volume of distribution is the apparent volume in which the drug is dissolved. Because the value of the volume of distribution does not have a true physiologic meaning in terms of an anatomic space, the term apparent volume of distribution is used.”; “The apparent volume of distribution is the primary distribution parameter for a drug. It is a constant for a drug and it is the ratio of the amount of drug in the body at any time to the plasma concentration at the same time. Note that although Vd has units of volume, it is important to recognize that it is a ratio and not a physiological volume.” [2-5]. The concept is therefore, old but since it was not of any relevance to physics, chemistry and engineering the property was never really studied systematically. Its use is gradually declining and the term apparent volume of distribution has (wrongly) become simply the volume of distribution.
What is really the apparent volume of distribution of a substance (Vd)? From the name alone, it seemed logical to seek to define it from a closed system solute two-phase distribution experiment, i.e., a solvent extraction method [6-8]. We had to look for a property that relates the mass of a solute added in the system to the concentration of the solute in one of the phases at equilibrium, while simultaneously providing information about its distribution in the other phase in the system.
Methods
Virtual solvent extraction experiments were designed in two immiscible phases that are in contact within a closed system. The volume of each phase was kept constant and the total volume was equal to the system volume (Vs). The affinity of the solute for phase 2 was set to be 16 times higher than phase 1 (partition coefficient, K=16 ). In order to study the apparent volume of distribution, a modified mass preservation equation (eq. 2) was used in which the final state is expressed in terms of the solute concentration and volume of phase 1, at equilibrium, instead of system concentration and system volume.
V1 ∙ C1 + V2 ∙ C2 = V d,1 ∙ C1 eq. 1 eq. 1
The product Vd,1 ∙ C1 must also equal the total mass of solute in the system, xs , where xs = x1+x2 (eq. 2 & 2a). The symbols x1, x2, xs and Vd,1 are the solute mass in phase 1, phase 2, total solute mass in the system, and the solute apparent volume of distribution with respect to phase 1, respectively.
We now have a property which we call the apparent volume of distribution of the substance with respect to phase 1 (V d,1) and relates the solute concentration in phase 1 to the total amount of solute in the system (eq. 2). Dividing eq. 1 with C1 yields another important equation indicating that the solute’s apparent volume of distribution is only dependent on the system phase volumes and the solute partition coefficient in the two phases (eq. 3). Similarly, the solute’s apparent volume of distribution with respect to phase 2 is equal to that shown in eq. 3a.
Results and Discussion
As with the partition coefficient, there are as many volumes of distribution and as many apparent volumes of distribution as the number of phases in a system. The volume of distribution of a solute in a phase is the volume of that phase in the system. On the other hand, the apparent volume of distribution of a solute associated with a phase in a two-phase system can be determined at equilibrium from the total mass of the solute in the system and its concentration in that phase or from the volumes of the phases and the partition coefficient. The smaller the partition coefficient for a phase, the larger the solute’s apparent volume of distribution associated with that phase. Eqs. 1-3 also indicate that although the apparent volume of distribution relates the total amount of solute in a system to a phase, its value only depends on the volumes of the two phases and the solute partition coefficient. We are now ready to provide a complete definition of theVd: the apparent volume of distribution associated with a solute in a phase can be defined as the phase volume that can form a solution with the total mass of the solute in the system while maintaining the solute concentration in that phase constant. For example, adding 500 g of solute into a system composed of 2400 L of phase 1 and 600 L of phase 2 with a partition coefficient K=16, results in Vd,1 = 12000 L (eq. 4), Vd,2 = 750 L, C1 = 0.04167 g/L, and C2 = 0.66667 g/L. Accordingly, in order to dissolve 500 g of the solute in phase 1 without causing any change in the original equilibrium solute concentration in that phase, you will have to add times bigger mass of solute (2500 g) into a system composed of
times larger phase volumes, that is, 12000 L of phase 1 in contact with 3000 L of phase 2. Obviously, changing the volumes of the two phases will result in a new apparent volume of distribution(V’d,1 = 60000 L). We can calculate the same solute concentration C1 using eq. 2, xs = 2500 g and the new value of V’d,1. Example 1 below, shows how to apply the concept of Vd in solvent extraction methods. We are assuming that the concentrations listed in Table 1 are measured in the organic phase, although this is a virtual solvent extraction experiment in which solute concentrations in the two phases were calculated as shown below with
Example 1
A compound is added in dry form in an extraction vessel containing 2,400 L of water (phase 1) and 600 L of immiscible organic solvent (phase 2). The compound concentration in the organic phase was measured as a function of total compound mass (xs) (Table 1). Answer the following:
Solution
The new method:
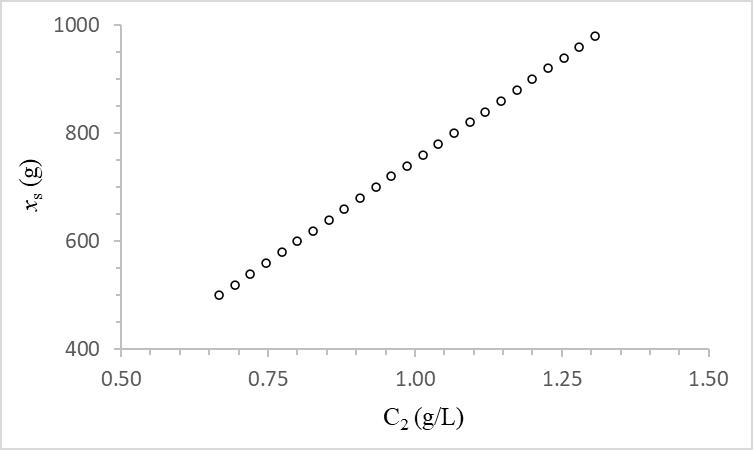
(a) Data of Table 1 was plotted in Figure 1. The system is unsaturated (linear) in the domain and range of compound concentration and total compound mass in the system. The solute’s apparent volume of distribution with respect to phase 2, Vd,2, is calculated from the slope of the equilibrium line or using eq. 2a to be equal to 750 L. The partition coefficient can be calculated
using eq. 3a and Vd,1 using eq. 3.
Notice that the extraction efficiency is the same everywhere in the given range of compound mass in the system.
Our answer is true assuming that the solvent system is still unsaturated after addition of 1.5 kg of compound.
(d) Caution! There will be a new apparent volume of distribution for a new phase volume and a new total system volume.
The traditional method:
Solving this problem, the traditional way is longer but we get the same answers. Most importantly, contrary to the previous method without knowing the value of the partition coefficient the problem cannot be solved (see eq. 4 below).
(b) First, we have to derive an expression of the fractional extraction efficiency. The extraction efficiency is defined as the percentage of solute that moves into the extracting solvent. Therefore, the fractional extraction efficiency after one extraction in the organic solvent (phase 2),f12is,
Substituting the above into the partition coefficient,
The denominator of the right-hand side of the above equation is Vd,2. Thus,
The ratio of the solute concentration is dictated exclusively by the value of the partition coefficient but the actual solute concentration in the two phases is also controlled by the phase volumes, which in turn, they control the value of the apparent volume of distribution. Therefore, at constant phase volumes, phase solute concentrations are directly proportional to the total mass of the solute in the system via the value of the apparent volume of distribution. This is why the apparent volume of distribution of a solute is such an important system property. By just looking at its value, you can tell where the solute is mostly located. For example, if the apparent volume of distribution of a solute with respect to a phase is larger than the whole system volume then most of the solute is in the other phase, the phase that was not sampled. In fact, you can determine exactly how much is in each phase using eq. 2. The linear plots of Fig. 1 indicate that at constant system volume and constant phase volumes, the apparent volume of distribution bears a constant value regardless of the total solute mass added in the system, and hence it is an intensive property that only depends on the type of matter. It can be defined as the volume of a phase that can contain the total mass of a solute added into the system while the phase solute concentration is maintained constant. That is, the phase volumes must vary to the same extent as the total mass of solute added into the system thus keeping C1constant (eq. 2). Each phase has its own solute apparent volume of distribution.
As we have seen in Example 1, one of the areas that the apparent volume of distribution has direct application is solvent extraction methods. The efficiency of an extraction method is evaluated by the fraction of the solute that moves into the extracting phase. Currently, calculating the extraction efficiency requires assessment of the partition coefficient from measurements of solute concentration in the two phases and knowledge of the volumes of the two phases. Using the concept of the apparent volume of distribution the extraction efficiency can be calculated from a single concentration measurement and the known volume of the assayed phase using eq. 4a. Alternatively, the capacity of a solvent to extract a substance can be evaluated by addition of increasing amounts of substance in the system. The apparent volume of distribution can be calculated from the slope of the line after linear regression analysis of measured phase solute concentrations as a function of solute mass in the system. Its value can then be used to optimize the product load in a solvent system in order to achieve a certain concentration of the substance in the extracting phase (Example 1c, New method). One of the advantages of the apparent volume of distribution is that it can be used to determine the partition coefficient in any biphasic system without having to measure the drug concentration in both phases. This is particularly useful in biological systems where the concentration of a drug cannot be measured in organs but the measured plasma drug concentration can be used to determine the amount of drug in the body’s organs, and in cells and other artificial systems where solute concentration measurement e.g., in the solid phase is very challenging.
Lastly, the apparent volume of distribution (Vd) is, similarly to the partition coefficient, a macroscopic equilibrium property of the system. It doesn’t matter what is contained in each phase and how complex is each immiscible phase in the system. All that the Vd needs is the solute amount in the system and the solute concentration in one of the phases composing the system, at equilibrium. It does not care about the path of solute distribution, solute interaction with other molecules in the system or how equilibrium is reached. The apparent volume of distribution of a substance can be determined in simple and complex systems alike as long as they are separated into communicating immiscible compartments. Similarly, its value can give information only about the solute concentration in two or more phases that are in contact within the system, at equilibrium.
Conclusions
The apparent volume of distribution was defined anew from solute distribution experiments. It can offer efficient and alternative ways to calculate the partition coefficient, amount of solute and phase solute concentrations in solvent extraction processes.
- Winkler AW, Smith P K (1938) The apparent volume of distribution of potassium injected intravenously. J Biol Chem 124: 589-98.
- “Volume of distribution”. en.wikipedia.org/wiki.
- Notari RE (1987) Biopharmaceutics and Clinical Pharmacokinetics: An Introduction, 4th ed., Marcel Dekker Inc.
- Shargel L, Yu ABC (2016) Applied Biopharmaceutics & Pharmacokinetics, 7th ed., McGraw-Hill.
- Rosenbaum S (2016) Basic Pharmacokinetics and Pharmacodynamics: An integrated textbook and computer simulations, 2nd ed., Wiley.
- Pradhan D, Sukla LB, Behera PK, Dash S (2021) Development of Bioleaching for extraction of metal values from Low-grade Ores and Wastes. Aspects in Mining & Mineral Science 7: 781-4.
- Zhang QW, Lin LG, Ye WC (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 13: 20.
- Dang HNP, Quirino JP, Shiyang C, Zhen S, Teng Z, et al. (2021) Computer-aided molecular design of solvents for chemical separation processes. Curr Opin Chem 35.

Tables at a glance
Figures at a glance