Taking Fruit Granola as a Snacks can Affect Post-Dinner Glucose Levels and Sleep Quality
Received Date: August 16, 2021 Accepted Date: August 16, 2021 Published Date: August 18, 2021
doi: 10.17303/jfn.2021.7.204
Citation:Hirofumi Masutomi (2021) Taking Fruit Granola as a Snacks can Affect Post-Dinner Glucose Levels and Sleep Quality. J Food Nutr 7: 1-16.
Abstract
Prolonged fasting is known to be a factor in blood glucose spikes, suggesting that a snacks may be useful in suppressing blood glucose spikes at dinner. Cereals are often eaten primarily at breakfast, and their effects as a snack are unknown. Therefore, we examined postprandial glucose level and sleep quality when eating fruit granola snacks prior to dinner. The subjects were recruited at Aikoku Gakuen Junior College and their snacking test schedule and dinner contents were randomly allocated. The subjects were involved in a crossover study (fruit granola as snacks, glucose snacks, no snack, and fruit granola added to dinner (no snack)) and glucose levels and sleep quality were measured. The fruit granola snack reduced the increase in postprandial glucose levels at the time of snack consumption compared with the glucose snack. The fruit granola snack was able to suppress the increase in postprandial glucose level at dinner compared to the glucose snack and no snack. In addition, the frequency of waking in the night was lower when the fruit granola snack was eaten compared to no snack. We found that eating fruit granola as a snack suppressed the increase in postprandial glucose levels after dinner, and possibly affected sleep quality.
Keywords:Glucose; Snack; Second Meal; Sleep Quality; Fruit Granola
Introduction
Various physiological phenomena such as body temperature, food intake, and the sleep–wake cycle are under the control of an endogenous circadian clock [1]. From studying the circadian clock, it is known that the timing of meals has an effect, and the academic field based on the function of food, nutrition, and the circadian clock is called chrononutrition [2]. A balanced diet is important for restricted feeding-induced entrainment of the peripheral clock rhythm, and balanced foods that lead to high blood glucose levels are suitable for peripheral clock entrainment. Mouse experiments showed that a better-balanced diet, rather than single nutrient intake, is more effective at resetting the circadian clock [3]. The idea of the second meal effect has become a topic of discussion in recent years. The second meal effect occurs when a first meal is consumed that slows the increase in blood glucose levels and reduces the sudden increase in blood glucose levels when a second meal is consumed [4]. Although the detailed mechanism of the second meal effect remains to be elucidated, a longer fasting time is associated with more glucose release into the blood, and its involvement in fatty acids causing insulin resistance has been reported [5,6].
When a blood glucose spike occurs, insulin is secreted, and the action of converting the carbohydrate to fat becomes excessive, leading to obesity [7]. According to the International Diabetes Federation Guidelines for the Management of Postprandial Glucose Levels, postprandial and post-load hyperglycemia are considered risk factors for vascular disease [8]. Inhibiting blood glucose spikes has also been implicated as an important factor for vascular disease because it affects prognosis [9]. Prolonged fasting is also known to be a factor in blood glucose spiking [10]. Previous studies have examined postprandial glucose variability after dinner by eating snack meals, showing that eating between lunch and dinner is associated with a slower increase in blood glucose after dinner compared with not eating snack meals, and stimulates good quality sleep [11]. Conversely, it has been reported that eating high-glycemic-index foods at dinner may worsen sleep quality [12].
The relationship between granola and blood glucose level has been reported. Granola is a general term for several types of grains, such as oats mixed with syrup and baked, which is eaten in Europe, the United States, and Japan. According to a systematic review of oat cereals, the average across 27 reports on the glycemic index of granola and muesli was 56 (range: 39–70) [13]. Postprandial blood glucose level and insulin levels were suppressed when granola was served as breakfast in an Asian population [14]. A randomized crossover double-blind study of a muesli containing 4 g of oat grain also reported the suppression of postprandial blood glucose levels compared to cornflakes [15].
However, granola, which is one of the cereals, is mainly eaten at breakfast, and there are few studies investigating the effects of eating fruit granola between lunch and dinner. Therefore, we examined postprandial glucose level and sleep quality when eating fruit granola snacks prior to dinner, focusing on active snacks.
Materials and Methods
Study Method
The subjects were recruited at Aikoku Gakuen Junior College and selected as follows: (1) healthy persons aged 18 years and older; (2) persons who provided written consent for participation. Excluded from the study were (1) individuals who were allergic to the food used in the study; (2) individuals with glucose intolerance (abnormal at medical examination for the past 2 years); (3) individuals with a fasting blood glucose of < 110 mg/dL; (4) individuals with a Body Mass Index (BMI) of >30; (5) patients with severe liver disease, kidney disease, heart disease, lung disease, psychiatric disease, blood disease, etc.; (6) individuals who took drugs such as antihypertensive drugs; (7) those who wished to become pregnant, or were pregnant/breastfeeding during the study; and (8) those who were judged by the study supervisor to be inappropriate for participation.
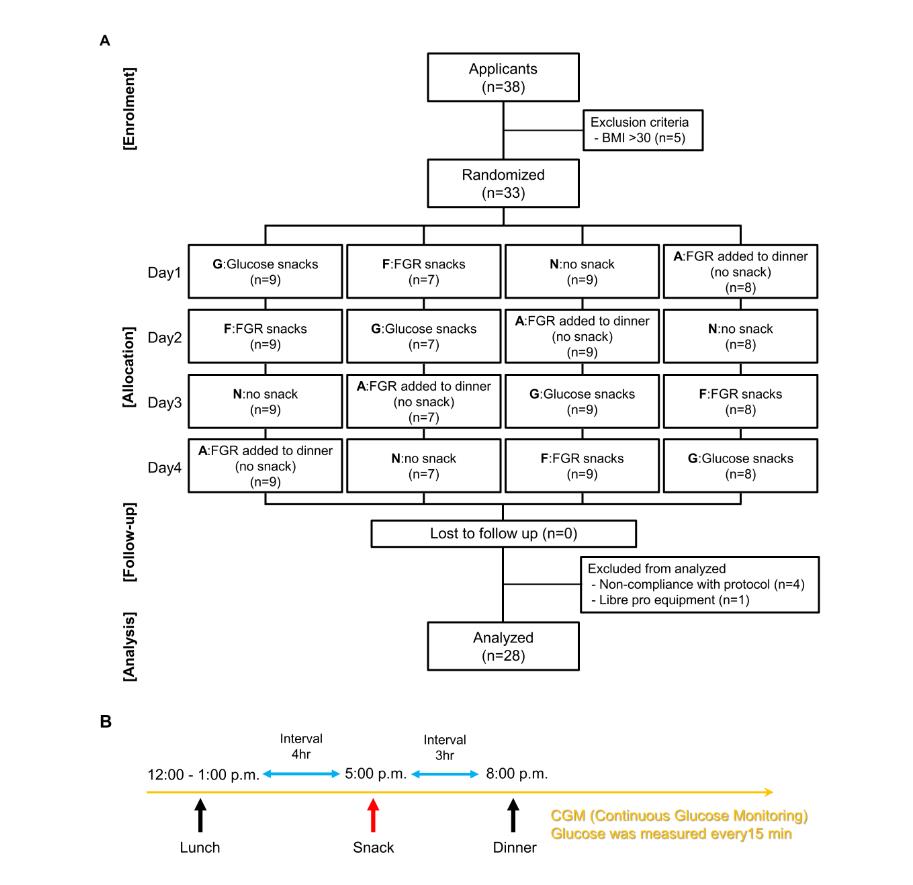
Publicly recruited subjects were randomly assigned (Table 1, Figure 1A, and Supplemental Table 1) for the study methods and dinner dietary content. The subjects were randomized by random number list and primary sponsor. In this study, we defined “snacks” that eat fruit granola or glucose at 5:00 p.m. Fruit granola (26 g, 114 kcal, Calbee Inc., Tokyo, Japan) and glucose (34 g, 114 kcal, Nippon Garlic Co, Gunma, Japan) were prepared for the snacks (Table 2). Glucose was weighed and 34.0 g was dissolved in 280 mL of water (Suntory, Tokyo, Japan) to be provided to subjects. Rice (Sato foods Co., Ltd., Niigata, Japan) and four kinds of main dishes (hamburger steak, simmered mackerel with miso, hamburger steak in cheese, and fried chicken) (Nichirei foods Inc., Tokyo, Japan) were prepared for dinner (Table 3). The dinner were prepared 551 kcal – 574 kcal and randomized to be unbiased. Supervision confirmed that the subject had completed the meal. The study was conducted over 14 days, and subjects wore a glucose meter (Freestyle Libre Pro; Abbott Japan LLC, Tokyo, Japan) and an activity meter to measure sleep quality (Fitbit Inspire HR; Fitbit, San Francisco, America) on the first day of the study. A over 1-day observation period was set for glucose monitoring stabilization [16] [17]. We set four randomized crossover trials after the run-in period as one cycle {fruit granola snacks (F), glucose snacks (G), no snack (N), and fruit granola added to dinner (no snack) (A)} (Table 1, Figure 1A, Supplemental Table 1). A group eat 26 g fruit granola as same F group. Subjects ate snacks at 5:00 p.m. and dinner at 8:00 p.m. (Figure 1B). The foods permitted lunch, snacks and dinner and subjects were fasting other meals from lunch until the next morning. The only drinks permitted were water or Japanese tea (0 kcal) and both were prohibited from lunch until the next morning. The study protocol was registered with the University-Hospital Health Information Network-Clinical Trials Registry System (UMIN-CRT) (registration number: UMIN000041223). This study was conducted with respect for the human rights of the participants, based on the Declaration of Helsinki, and with approval from the Ethics Review Committee of Aikoku Gakuen Junior College (approval number: 2019-R04). Subjects were verbally informed of the outline, purpose, investigation content, protection of personal information, etc., of this study, and gave written consent. In doing so, they were also told that all data obtained were coded and individuals could not be identified, that data were analyzed in a population, that they would not be used for purposes other than research, that subjects would not be disadvantaged, and that they would be free to participate in the research or not.
Glucose levels
The glucose levels of the subjects were monitored using FreeStyle Libre Pro. FreeStyle Libre Pro is an instrument capable of measuring interstitial glucose levels every 15 minutes for 14 consecutive days. Blood glucose levels from fingertips and interstitial blood glucose levels were reported to be correlated [18]. The FreeStyle Libre Pro device was placed on the upper arm of the subject by a physician. After completion of the study, the devices were retrieved and data were read by a dedicated device. Three-item analyses were conducted: (1) glucose level every 15 minutes after snack and dinner consumption; (2) maximal glucose level change; (3) area under the curve (AUC). The glucose levels were monitored and analyzed every 15 minutes from 5:00 p.m. to 3:00 a.m. The maximum change in glucose level at snack was set to 0 at 5:00 p.m. and the amount of change was analysis from 5:00 p.m. to 8:00 p.m. The AUC at snacks was accumulated from 5:00 p.m. to 8:00 p.m. The maximum change in glucose level at dinner was set to 0 at 8:00 p.m. and the amount of change was analysis from 8:00 p.m. to 1:00 a.m. The AUC at dinner was accumulated from 8:00 p.m. to 1:00 a.m. The AUC was calculated by the trapezoidal method [19].
Questionnaire Survey
A questionnaire survey was administered that consisted of five items to assess the subjects: feeling hungry until dinner, concentration until dinner, goodness of subjective sleep, subjective deep sleep, and good subjective awakening in the morning. The Likert scale was used for the questionnaire survey, and it was created based on the five guidelines for constructing measurement means using the Likert scale [20] [21]. In this study, a point formula evaluation of 1 to 5 points was adopted, and the subjects were instructed to enter the points the day after the test. This evaluation method was analyzed by a nonparametric test because it is unclear whether the respondents felt that the intervals between adjacent options were evenly spaced across 5 points [22].
Sleep Quality
The Fitbit Inspire HR (Fitbit, San Francisco, America) was used to measure sleep quality. The Fitbit is a type of activity tracker, and it is possible to measure the quality of sleep (sleep onset time, stage of sleep, wakefulness, etc.) from the heart rate measurements taken by the device [23]. Subjects were instructed to wear the Fitbit consistently, including when sleeping, for 14 consecutive days. Fitbits were then retrieved after the completion of the study and an analysis tool dedicated to Fitbit was used. In this study, the following five items were analyzed: (1) the percentage of waking hours during sleep (%); (2) the percentage of rapid eye movement (REM) sleep (%); (3) the percentage of non-REM sleep (light); (4) the percentage (%) of non-REM sleep (deep); (5) the number of awakenings during sleep.
Statistical Analysis
A two-way repeated ANOVA was used to analyze hourly glucose data, and Dunnett’s test was used for multiple comparisons. An one-way repeated ANOVA was used for the analysis of maximal glucose change, AUC, and sleep quality, and Dunnett’s test was used for multiple comparisons. The questionnaire-based survey used Friedman ranking, and the Dunn method was used for multiple comparisons. We used G power to determine the sample size. Graph Pad Prism 8.4.3 was used as the statistical software for analysis, and the level of statistical significance was set at p < 0.05.
Results
Screening
Among the participants who volunteered to participate, 38 agreed. The internal exclusion criterion, BMI of >30, excluded five individuals (Figure 1A). Within this protocol, non-adherence was found for four persons, and the inability to measure glucose level due to poor measurement instrument performance occurred for one person. In total, the results from 28 participants were analyzed. There were no adverse events in this study. All subjects were women, with a mean age of 32.0 ± 13.6 years (range: 19–64 years) and BMIs of 22.3 ± 3.2 (range: 17–29). The average value of two tests was adopted for glucose levels and the questionnaire to ensure reproducibility.
Glucose levels
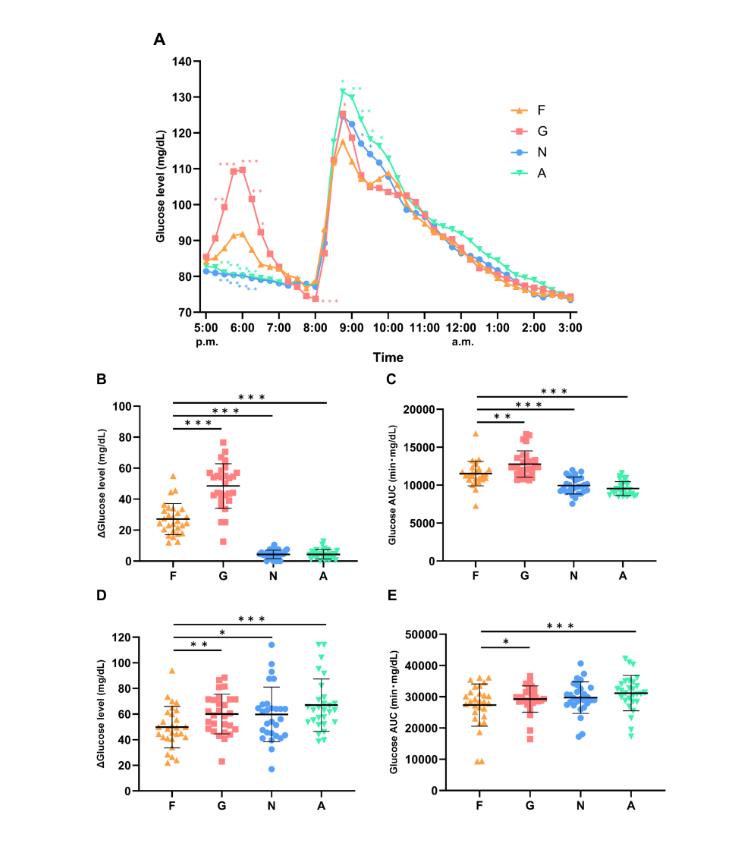
At first, when we examined the changes in glucose levels during the snack meals, the postprandial glucose levels in the F and G groups were elevated (Figure 2A, Supplemental Table 2). The breadth of the glucose increase during the dinner meal in group F was suppressed compared to group G (F:26.5±10.1, G:47.3±14.4) (Figure 2A, Supplemental Table 2). Glucose levels in groups N and A (no snack meals) were not elevated. Compared with the change in maximal glucose level during the meal, group F showed a lower level and a suppressed increase in glucose level compared with group G (Figure 2B, Supplemental Table 3). The AUC similarly showed lower levels and inhibition of the increase in glucose levels compared to group G (F:11526 ± 1616, G: 12781±1742) (Figure 2C, Supplemental Table 3).
Next, when analyzing the glucose level variability at dinner, the pre-dinner glucose level in group G was significantly lower than in group F at 8:00 p.m. (F:78.9±10.0, G:73.7±10.2) (Figure 2A, Supplemental Table 2). The postprandial glucose level in group G was significantly higher compared to group F at 8:45 p.m. (F:117.6±20.0, G:125.3±24.8). The postprandial glucose level in group N was significantly higher than in group F at 9:15 and 9:30 p.m. (9:15; F:107.2±19.6, N:117.1±26.7, 9:30; F:105.6±18.1, N:114.1±26.6). Postprandial glucose levels in group A were significantly higher than in group F at 8:45, 9:00, 9:15, 9:30, and 9:45 p.m. (A: 8:45;131.5±31.2, 9:00;129.8±31.5, 9:15;123.7±29.0, 9:30;118.2±25.9, 9:45;116.4±20.8). The maximal glucose level change in group F was significantly lower than in groups G, N, and A, and the group F increase in postprandial glucose level was suppressed (F:48.6 ± 16.2, G:58.5 ± 15.4, N:58.4 ± 21.4, A:65.4 ± 20.4) (Figure 2D, Supplemental Table 3). For the AUC at dinner, group F showed significantly lower values compared with groups G and A (F:27352±6770, G: 29277±4223, N: 29730±5053, A: 31162±5654) (Figure 2E, Supplemental Table 3).
Questionnaire Survey
When analyzing the questionnaire survey regarding hunger and concentration until dinner, hunger in group F was lower compared to groups G, N, and A (Supplemental Figure 1, Supplemental Table 4). Concentration was also significantly higher in group F than in groups G, N, and A.
When analyzing the questionnaire survey regarding sleep, the subjective quality of sleep was better in group F compared to group A. For subjective depth of sleep, group F reported higher values compared to group N. Good subjective awakening showed higher values in group F compared to groups N and A.
Sleep Quality
When analyzing sleep quality as measured by the Fitbits, the percentage of waking hours during sleep, the percentage of rapid eye movement (REM) sleep, and the percentages of non-REM sleep (light, deep) were not significantly different between groups (waking; F:45.9 ± 62.3, G:37.7 ± 72.5, N:54.1 ± 57.6, A:35.5 ± 40.7. REM; F:26.0 ± 19.9, G:29.7 ± 15.0, N:27.5 ± 19.2, A:20.6 ± 14.9. non-REM (light); F:45.9 ± 62.3, G:37.7 ± 72.5, N:54.1 ± 57.6, A:35.5 ± 40.7. non-REM (deep); F:26.0 ± 19.9, G:29.7 ± 15.0, N:27.5 ± 19.2, A:20.6 ± 14.9) (Figure 3A–D, Supplemental Table 5). The number of awakenings during sleep was significantly lower in group F compared to groups N and A (F:1.6 ± 1.2, G:2.8 ± 2.1, N:3.0 ± 1.8, A:3.2 ± 2.5) (Figure 3E, Supplemental Table 5).
Discussion
In this study, we found that (1) the fruit granola snack significantly suppressed the increase in postprandial glucose level at the time of snack consumption compared with the glucose snack; (2) the postprandial glucose rise with dinner was significantly suppressed in the fruit granola snack group compared with groups G, N and A; (3) a significantly lower number of awakenings occurred during sleep in the fruit granola snack group than in groups N and A (no snack).
The postprandial glucose level at the time of the snacks was significantly suppressed in the fruit granola group compared to the glucose group (Figure 2A–C). The caloric counts of the fruit granola and glucose snacks were the same, at 114 kcal, but the increase in glucose levels was likely suppressed in the fruit granola group due to differences in the carbohydrate content (Table 2). Cereals that suppress elevated blood glucose levels include foods high in dietary fiber, such as barley and oats. Dietary fiber includes insoluble dietary fiber and water-soluble dietary fiber. Water-soluble dietary fiber is highly viscous and has been reported to slow the rate of movement of food from the stomach to the small intestine and the rate of digestion of glucoses in the small intestine, and to gently increase blood glucose levels [24]. Fruit granola (26 g per serving) contains 2.7 g of dietary fiber, plus β glucan, another water-soluble dietary fiber. Oat β-glucan was reported to have a mild effect on glucose absorption, and it is possible that the fruit granola group experienced a slower increase in postprandial blood glucose level due to the intake of dietary fiber [25].
A fruit granola snack may be a valuable instrument to suppress postprandial glucose elevation at dinner (Figure 2A, D–E). In a study by Kuwahara, et al., a snack of biscuits containing leaves of morula and barley 4 hours prior to dinner suppressed the increase in postprandial blood glucose levels during dinner [26]. A report stated that the longer the fasting time, the higher the blood glucose level [10]. In this study, the environment in which the post-dinner increase in glucose was measured must be considered, because the fasting time was 3 hours longer without meals than with snacks. Therefore, groups N and A (without the snack) experienced increased postprandial glucose level at dinner compared with the fruit granola snacks group. For groups G and F (which subsequently ate snacks at 5:00 p.m.), group G had significantly higher maximal glucose levels at dinner than group F (Figure 2A, D). As mentioned above, fruit granola contains a large amount of dietary fiber. In water-soluble dietary fiber, such as β glucan, there is a direct glucose absorption suppression effect by viscosity and an indirect suppression of blood glucose increase through intestinal bacteria [27] [28]. Intestinal bacteria in the large intestine grow on the basis of water-soluble dietary fiber and produce short-chain fatty acids such as butyrate and acetic acid [28]. Short-chain fatty acids stimulate L cells in the small intestine and gut to secrete hormonal glucagon-like peptide (GLP) -1 [29]. GLP-1 can stimulate the secretion of insulin, and the second meal effect in which the sustained insulin secretion before dinner suppresses the postprandial blood glucose rise after dinner was considered [30]. In addition, glucose levels in the glucose group at the beginning of dinner were significantly lower than those in the fruit granola snack group (Figure 2D). Differences in glucose levels at the start of dinner may also be one of the factors that resulted in differences in the maximal glucose changes between the glucose and fruit granola snack groups. From the above, it seems to be desirable to consume foods high in dietary fiber as snacks. In addition, the blood monitoring of insulin should be examined as a future problem, because insulin secretion seems to strongly affect the second meal effect.
The glycemic index of granola and muesli is 56 (range: 39–70) [13]. When granola was served as a snack compared to glucose, postprandial blood glucose level and insulin levels were also suppressed [31]. In addition, there was a correlation between blood glucose level and insulin level in the second meal effect when eating a snack high in dietary fiber [32]. The postprandial glucose level at the time of the snacks and dinner was significantly suppressed in the fruit granola snack group compared to other groups (Figure 2A-B,D). We perhaps that the insulin level decreased in the participants of this study. Insulin is a hormone that lowers blood glucose levels, and insulin monitoring is extremely important for understanding blood glucose fluctuations. The inability to measure insulin is a limitation of this study.
From the questionnaire, the fruit granola snack group experienced low hunger before dinner and high concentration (Supplemental Figure 1A-B). Previous studies have reported that a lack of energy supplementation with breakfast is associated with a stronger sense of hunger and reduced work efficiency in subjects [33]. Groups N and A, which did not eat a snack, had low glucose levels, and because energy supplementation was not possible, the results show a strong sense of hunger and low concentration. Subsequently, in the glucose-to-glucose diet, a sharp increase in blood glucose was observed after glucose ingestion. Hypoglycemic states caused by large amounts of insulin have been reported to cause hunger, irritation, sleepiness, insomnia, and decreased concentration [33]. Insulin secretion rises according to the sudden increase in the blood glucose level. Therefore, we found that group G reported stronger hunger and less concentration than group F in this study. The Likert scale is a valid evaluation method, but it is also subjective. As such, the answers provided may not be truly reflective of the respondents.
The fruit granola snack group reported significantly less frequent awakenings during sleep. Sleep is divided roughly into REM sleep and non-REM sleep; in addition, stages I–IV exist for non-REM sleep depending on the depth of the sleep (stages 1–2 are shallow sleep, stages 3–4 are deep sleep) [34]. Within this stage, it is the deep non-REM sleep of stage IV that recovers the fatigue of the brain. It has been reported that the processing of waste products in the brain involves not only blood flow, but also cerebrospinal fluid flow, and that processing is mostly performed during non-REM sleep. In this study, no changes were found in the percentages of REM sleep and non-REM sleep duration between the groups (Figure 3A–D). Wakefulness during sleep has been reported to interfere with good sleep quality [35]. A study also reported that eating between lunch and dinner is associated with a slower increase in glucose levels after dinner compared with no meal [26]. Eating snacks between lunch and dinner is associated with a slower increase in blood glucose after dinner compared with not eating snacks, and stimulates good quality sleep [11]. We found a significantly lower number of awakenings during sleep in group F of eating fruit granola snacks (Figure 3E). The lower number of awakenings during sleep in group F may have influenced the outcome of subjective sleeping assessments (Supplemental Figure 1C–E). Nevertheless, since the survey was a subjective evaluation, it may contain bias.
Conclusions
We found that fruit granola as a snacks may suppress the increase in postprandial glucose levels at dinner. In addition, the frequency of awakenings during sleep was lower in the fruit granola snack group compared to groups with no snack, and the possibility of affecting sleep quality was also indicated. These results are expected to contribute to the development of blood glucose control and sleep research by snacks in the future.
Author Contributions
Conceptualization, HM and AF; methodology, HM, KI, KH, and AF; validation, HM and AF; formal analysis, HM and AF; investigation, HM and AF; writing and editing, HM, AF; supervision, KI and KH; project administration, AF. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
HM, and KI are employees of Calbee, Inc. KH and AF reports no conflicts of interest in this work.
Sources of Funding
The study was conducted with funding from Calbee, Inc., Tokyo, Japan.
Acknowledgement
The authors would also like to thank MDPI English Editing for English language editing.
Supplemental
- King DP, Takahashi JS (2000) Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci 23: 713-42.
- Oike H, Oishi K, Kobori M (2014) Nutrients, Clock Genes, and Chrononutrition. Curr Nutr Rep 3: 204-12.
- Hirao A, Tahara Y, Kimura I, Shibata S (2009) A balanced diet is necessary for proper entrainment signals of the mouse liver clock. PLoS One 4: e6909.
- Jenkins DJ, Wolever TM, Taylor RH, Griffiths C, Krzeminska K, et al. (1982) Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr 35: 1339-46.
- Wolever TM, Bentum-Williams A, Jenkins DJ (1995) Physiological modulation of plasma free fatty acid concentrations by diet. Metabolic implications in nondiabetic subjects. Diabetes Care 18: 962-70.
- Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR (1988) Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr 48: 1041-7.
- McClain DA (2002) Hexosamines as mediators of nutrient sensing and regulation in diabetes. J Diabetes Complications 16: 72-80.
- Parkin CG, Buskirk A, Hinnen DA, Axel-Schweitzer M (2012) Results that matter: structured vs. unstructured self-monitoring of blood glucose in type 2 diabetes. Diabetes Res Clin Pract 97: 6-15.
- Brand-Miller J, Dickinson S, Barclay A, Celermajer D (2007) The glycemic index and cardiovascular disease risk. Curr Atheroscler Rep 9: 479-85.
- Kajiyama S, Imai S, Hashimoto Y, Yamane C, Miyawaki T, et al. (2018) Divided consumption of late-night-dinner improves glucose excursions in young healthy women: A randomized cross-over clinical trial. Diabetes Res Clin Pract 136: 78-84.
- Imai S, Kajiyama S, Hashimoto Y, Nitta A, Miyawaki T, et al. (2018) Consuming snacks mid-afternoon compared with just after lunch improves mean amplitude of glycaemic excursions in patients with type 2 diabetes: A randomized crossover clinical trial. Diabetes Metab 44: 482-7.
- Afaghi A, O’Connor H, Chow CM (2007) High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr 85: 426-30.
- Tosh SM, Chu Y (2015) Systematic review of the effect of processing of whole-grain oat cereals on glycaemic response. Br J Nutr 114: 1256-62.
- Tan WSK, Tan WJK, Ponnalagu SD, Koecher K, Menon R, et al. (2018) The glycaemic index and insulinaemic index of commercially available breakfast and snack foods in an Asian population. Br J Nutr 119: 1151-6.
- Hlebowicz J, Darwiche G, Bjorgell O, Almer LO (2008) Effect of muesli with 4 g oat beta-glucan on postprandial blood glucose, gastric emptying and satiety in healthy subjects: a randomized crossover trial. J Am Coll Nutr 27: 470-5.
- Takashi YAMAWAKI NH, Jie ZHANG, Eri YAMAGUCHI, Hiroshi KOMURO (2019) Glucose Profiles Analysis Using the Free Style Libre Pro® in 3 Cases of Total Gastrectomy Without Hypoglycemic Symptoms. J Japanese Asso Rural Med 68: 64-70.
- Hoss U, Budiman ES (2017) Factory-Calibrated Continuous Glucose Sensors: The Science Behind the Technology. Diabetes Technol Ther 19: S44-S50.
- Kumagai R, Muramatsu A, Fujii M, Katakura Y, Ito K, et al. (2019) Comparison of glucose monitoring between Freestyle Libre Pro and iPro2 in patients with diabetes mellitus. J Diabetes Investig 10: 851-6.
- Nakajima H (2012) Diurnal variation in blood glucose response to rice intake. J Osaka Aoyama University, 5: 7-15.
- Likert R (1932) A technique for the measurement of attitudes. Archives of Psychology 22.
- Nemoto T, Beglar D (2014) Developing Likert-scale questionnaires. JALT2013 Conference Proceedings.
- Buysse DJ (2014) Sleep health: can we define it? Does it matter? Sleep 37: 9-17.
- Liang Z, Chapa-Martell MA (2019) Accuracy of Fitbit Wristbands in Measuring Sleep Stage Transitions and the Effect of User-Specific Factors. JMIR Mhealth Uhealth 7: e13384.
- Tabatabai A, Li S (2000) Dietary fiber and type 2 diabetes. Clin Excell Nurse Pract 4: 272-6.
- Granfeldt Y, Nyberg L, Bjorck I (2008) Muesli with 4 g oat beta-glucans lowers glucose and insulin responses after a bread meal in healthy subjects. Eur J Clin Nutr 62: 600-7.
- Kuwahara M, Kim HK, Ozaki M, Nanba T, Chijiki H, et al. (2020) Consumption of Biscuits with a Beverage of Mulberry or Barley Leaves in the Afternoon Prevents Dinner-Induced High, but Not Low, Increases in Blood Glucose among Young Adults. Nutrients 12.
- Angelov A, Yaneva-Marinova T, Gotcheva V (2018) Oats as a matrix of choice for developing fermented functional beverages. J Food Sci Technol 55: 2351-60.
- Miyamoto J, Watanabe K, Taira S, Kasubuchi M, Li X, et al. (2018) Barley beta-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice. PLoS One 13: e0196579.
- Hernandez MAG, Canfora EE, Jocken JWE, Blaak EE (2019) The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 11.
- Johansson EV, Nilsson AC, Ostman EM, Bjorck IM (2013) Effects of indigestible carbohydrates in barley on glucose metabolism, appetite and voluntary food intake over 16 h in healthy adults. Nutr J 12: 46.
- Shively CA, Apgar JL, Tarka SM, Jr. (1986) Postprandial glucose and insulin responses to various snacks of equivalent carbohydrate content in normal subjects. Am J Clin Nutr 43: 335-42.
- Rebello CJ, Johnson WD, Pan Y, Larrivee S, Zhang D, et al. (2020) A Snack Formulated with Ingredients to Slow Carbohydrate Digestion and Absorption Reduces the Glycemic Response in Humans: A Randomized Controlled Trial. J Med Food 23: 21-8.
- Higuchi T HK, Imazuya S, Irie S (2007) The effect of breakfast omission and breakfast type on body temperature, mood and intellectual performance. Nihon Rinsho-Eiyo gakkaizasshi 29: 35-43.
- Hori T, Sugita Y, Koga E, Shirakawa S, Inoue K, et al. (2001) Proposed supplements and amendments to ‘A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin Neurosci 55: 305-10.
- Cole CS, Richards KC (2006) Sleep in persons with dementia: Increasing quality of life by managing sleep disorders. J Gerontol Nurs 32: 48-53.


Tables at a glance
Figures at a glance