LGR5 Expression in Oral Squamous Cell Carcinoma and its Prognostic Value
Received Date: May 29, 2020 Accepted Date: June 19, 2020 Published Date: June 22, 2020
doi: 10.17303/jdoh.2020.7.103
Citation:Amirah Alnour (2020) LGR5 Expression in Oral Squamous Cell Carcinoma and its Prognostic Value. J Dent Oral Health 7: 1-6.
Abstract
Accumulated numbers of papers mention the role mechanism of neoplasm process and factors contributed to this entity. Many factors that may contribute to the initiation of tumors have been studied, mainly stem cells. We are going to review the results of that studies related to oral squamous cell carcinoma (OSSC), as the most common cancer in the oral cavity. The most relevant cause of OSSC is the heavy smoking of tobacco and alcohol consumption. In addition to other risk factors such as lifestyle. Many biomarkers have been detected and studied as to their potential role in the pathogenesis of this carcinoma. One of those factors is LGR5, which is one of the novel markers that label stem cells. LGR5 is also a literary known as Leucine-rich repeat-containing G-protein coupled, it is a well-known marker of stem cells in several tissue. In literature, many news papers refer to the possible role of this LGR5 in many variants of cancers in different organs, but there is not enough data according to the role of LGR5 in oral cancers in general, and the possible prognostic factor of it as well.
Keywords: LGR5; Oral; Carcinoma; Neoplasm; Tumor
Introduction
According to the epidemiologic studies released by the World Health Organization, cancers have been proven to raise the rate of mortality more than heart diseases and stroke [1,2].
Oral squamous cell carcinoma is considered as the most common malignancy in the oral cavity. Its prevalence tends to occur in developing countries, especially south-central Asia [3, 4].
In the oral cavity, squamous cell carcinoma is more attributed to the heavy smoking of tobacco and alcohol consumption. In addition to other risk factors such as lifestyle, a diet rich in fat and lack of fruit and vegetables, and low socioeconomic status [5].
Death associated with these cancers reaches 292000 ( about 3.6%). In 2030 it will reach 856000 cases [6].
Many biomarkers have been proved to play an important role in the pathogenesis of this carcinoma. These biomarkers have a prognostic and/or a therapeutic value as to the oral squamous cell carcinoma.
Previous studies mentioned many biomarkers that have contributed to this initiation such as cyclin D1, fibroblast growth factor receptor (FGFR1), epidermal growth factor receptor (EGFR), BCL2, E-cadherin, P53, c-MYC, COX-1 and LGR5, and many others [7-9].
Yet the presence of cancerous stem cells remains the main cause of recurrence after treatment. And extirpation of those stem cells assures the long-lasting cure of cancer [10].
Biologically, stem cells are known as cells that have the ability to differentiate into any mature cells of a specific tissue [11, 12]. Accumulated studies have been carried out in order to recognize and identify markers of stem cells as well as their role in the recurrence of variant types of tumors, mainly because of their property of self-renewal through differentiation. Though these cells could be used in the purpose of therapeutic fields, especially cancers [13, 14].
Obvious evidence suggested that stem cells exist in the hematopoietic system and these have the ability to give rise to several tissues specific stem cells or progenitor cells [15, 16].
Wnt pathway has been revealed as an essential regulator of stem cells. Activation of this pathway is highly associated with cancer pathogenesis. The self-renewal ability of stem cells and/or progenitor cells is mediated by the Wnt pathway and this mechanism is destructed in cancerous cells which is the main key in malignant transformation [ 17-19].
LGR5 is one of the markers that label stem cells. LGR5 or what is literary known as Leucine-rich repeat-containing G-protein coupled (it is also referred to as Gpr49), is a wellknown marker of stem cells in several tissue. This protein is involved in the canonical Wnt signaling pathway. Its main function is to maintain adult intestinal stem cells during postembryonic development [20, 21].
It is also expressed in rare cells of several tissues such as the stomach, intestinal stem cells, and secondary germ of the telogen hair follicle [22, 23].
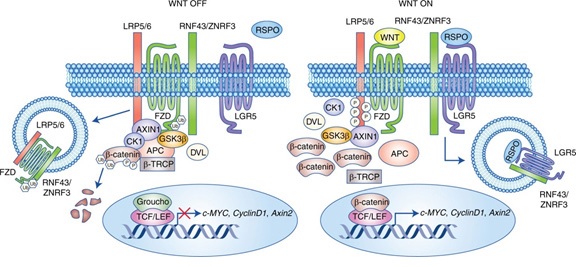
As it has been mentioned above, stem cells in the skin and intestinal epithelium are regulated by the Wnt pathway. In this pathway Wnt bind to frizzled/LRP co-receptor complex that will activate the canonical signaling pathway. This will activate the canonical signaling pathway and result in the inactivating of APC destruction complex and as a result β-catenin stabilization. β-catenin then translocates to the nucleus where it binds TCF transcription factors and activates Wnt-target gene LGR5. LGR5 in turn, encode seven membranous proteins for coupling to G-protein [24, 25] (figure 1).
LGR5 gene is proved to be expressed in other cancers such as oral, liver, lung, colon, and ovary [26, 27].
It has been revealed that LGR5 homozygous distribution resulted in neonatal lethality, ankyloglossia, ingestion of air at birth, and gastrointestinal tract dilation [28, 29].
An interesting result showed that LGR5 is selectively expressed in the taste bud tissue of the posterior tongue. LGR5 is strongly expressed in cells at the base of circumvallate and foliate papillae and weakly expressed in the basal area of taste buds [30, 31].
Stem cells that are restricted in tissue microenvironment are called niches. Signals that are generated from these cells, control cell cycle, maintain cell-renewal or going through differentiation [32].
In another word, niches also play a critical role in the fate of daughter cells, to remain as stem cell and keep stem cells pool (preventing cancer formation as well) or to differentiate [33].
Wnt signaling pathway plays an important role in activation or silencing the proliferation of stem cells reservoirs. Dis-activation of Wnt signaling by removal of Tcf4 or β-catenin or by activation the Wnt inhibitors leads to end any proliferation activity. So what happens in cancer formation is the activation of the Wnt pathway through several mutations within the cell [23].
Tcf4 is a transcription factor, when bonding with β-catenin, activates expression of the Wnt gene. So in absence of this factor cell proliferation will stop.
In the cell nucleus, stabilized β-catenin binds with the Tcf transcription factor, which in turn leads to Wnt-target gene transcription. Tcf interacts with SMAD4 which will cause the connection of Wnt and BMP signaling pathways. The phosphorylation process of Tcf by Nemo-like kinase or the interaction between β-catenin and ICAT will badly trigger the Wnt pathway [34, 35].
LGR5 expression in other oral lesions:
LGR5 expression was found to be associated with increased potential of malignancy in oral epithelial dysplasia. Andrew, et al. suggested that LGR5 was restricted to the basal layer of normal oral epithelium. Then it became intense in severe dysplasia and uniformly diffused through poorly differentiated oral squamous cell carcinoma [36].
Boddupally mentioned in his research that LGR5 positive stromal stem cells have merits of neural crest stem cells including clonal growth and multipotential differentiation. As LGR5 plays an important role in taste bud differentiation, this study suggested its role in tongue wound healing [37].
In general, markers of stem cells in oral cancers (CD44, CD34, CD29, BMI, LGR5, LGR4,…..etc.) remain targets for novel therapeutic strategies to reduce cancer stem cells in various cancers, including oral cancers [38].
Critical evaluation of potential & limitation of employing biomarkers of cancer stem cell as prognostic factors:
Previous studies suggested many biomarkers that play a remarkable role as cancer stem cells (CSCs) in squamous cell carcinoma initiation and other tumors as well.
These factors include; CD34, CD24, CD133, CD44, CD29, CD 39, BMI1, PDPN, LGR5,LGR, ABCG2, dentin sialophosphoprotien (DSPP)….and others.
The last factor has been correlated to tumor behavior (aggressiveness and prognosis). Interestingly, silencing DSPP has been associated with the decreased potential of oral neoplasia.
Also, BMI1, PDPN, and CD133 have been expressed in most head and neck squamous cell carcinomas (HNSCCs) in the invasion front.
Tumor-associated macrophages (TAMs) are connected with markers of cancer stem cells and influence the survival of oral squamous cell carcinoma (OSCC), suggesting to play a critical role as a potential prognostic factor in OSCC.
These two factors are studied as a component of the tumor microenvironment, and this microenvironment plays an important role in tumor development.
Macrophages have two main types: M1: which has anti-tumor features whereas M2 triggers invasion and metastasis. In neoplasia, TAMs tend to possess an M2-like phenotype.
Some biomarkers that are associated with TAMs are CD 68 and CD 163.
CD68 is used to recognize TMAs. While CD163 was suggested to mark the poor prognosis and decreasing overall survival in many cancers.
Conclusion
Because of the expression of LGR5 in variant organs, it may represent a global marker of stem cells. The role of LGR5 was explained in the tumorgenesis, by activating (with other stem cells) the Wnt signaling pathway, which would cause cytoplasmic β-catenin to be accumulated. The last progress is associated with tumoegenesis.
There are not enough studies on the role of LGR5 in oral squamous cell carcinoma. More researches are needed to be done in this context and to be linked to the metastasis of this carcinoma in order to define its role as a prognostic and/or therapeutic markers for this carcinoma.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. (2015) Cancer incidence and mortality worldwide: sources, methods, and major patterns in GLOBOCAN 2012. International journal of cancer 136: E359-E386.
- Petersen PE (2009) Oral cancer prevention and control–the approach of the World Health Organization. Oral oncology 45: 454-460.
- Tandon P, Dadhich A, Saluja H, Bawane S, Sachdeva S (2017) The prevalence of squamous cell carcinoma in different sites of the oral cavity at our Rural Health Care Centre in Loni, Maharashtra–a retrospective 10-year study. Contemporary Oncology 21: 178.
- Gupta N, Gupta R, Acharya AK, Patthi B, Goud V, Reddy S, et al. (2016) Changing Trends in the oral cancer-a global scenario. Nepal journal of epidemiology 6: 613.
- Mashberg A, Boffetta P, Winkelman R, Garfinkel L (1993) Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among US veterans. Cancer 72: 1369-1375.
- GLOBOCAN I (2012) Estimated cancer incidence, mortality, and prevalence worldwide in 2012. International Agency for Research on Cancer. World Health Organization Press Release.
- Koole K, Brunen D, Van Kempen PM, Noorlag R, De Bree R, Lieftink C, et al. (2016) FGFR1 is a potential prognostic biomarker and therapeutic target in head and neck squamous cell carcinoma. Clinical Cancer Research. 22: 3884-3893.
- Akervall J, Nandalur S, Zhang J, Qian C-N, Goldstein N, Gyllerup P, et al. (2014) A novel panel of biomarkers predicts radioresistance in patients with squamous cell carcinoma of the head and neck. European journal of cancer. 50: 570-581.
- Smith BD, Smith GL, Carter D, DiGiovanna MP, Kasowitz KM, Sasaki CT, et al. (2001) Molecular marker expression in oral and oropharyngeal squamous cell carcinoma. Archives of Otolaryngology-Head & Neck Surgery 127: 780-785.
- Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. New England Journal of Medicine 355: 1253-1261.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. cell 131: 861-872.
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. (2003) Identification of a cancer stem cell in human brain tumors. Cancer research 63: 5821-5828.
- Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumors: accumulating evidence and unresolved questions. Nature reviews Cancer 8: 755.
- Chen K, Huang Y-h, Chen J-l (2013)Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacologica Sinica 34: 732.
- Bianco P, Robey PG (2000) Marrow stromal stem cells. The Journal of clinical investigation 105: 1663-1668.
- Weissman IL (2000) Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science 287: 1442- 1446.
- Polakis P (2000) Wnt signaling and cancer. Genes & development 14: 1837-1851.
- Reya T, Clevers H (2005) Wnt signaling in stem cells and cancer. Nature 434: 843.
- Barker N, Clevers H (2006) Mining the Wnt pathway for cancer therapeutics. Nature reviews Drug discovery 5: 997.
- Muñoz J, Stange DE, Schepers AG, Van De Wetering M, Koo BK, Itzkovitz S, et al. (2012) The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+ 4’cell markers. The EMBO journal 31: 3079-3091.
- Schepers AG, Snippert HJ, Stange DE, et al. (2012) Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337: 730-735.
- Jaks V, Barker N, Kasper M, Van Es JH, Snippert HJ, et al. (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature genetics 40: 1291.
- Haegebarth A, Clevers H (2009) Wnt signaling, lgr5, and stem cells in the intestine and skin. The American journal of pathology 174: 715-721.
- Chen Q, Cao H-Z, Zheng P-S (2014) LGR5 promotes the proliferation and tumor formation of cervical cancer cells through the Wnt/β-catenin signaling pathway. Oncotarget 5: 9092.
- White BD, Chien AJ, Dawson DW (2012) Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 142: 219-232.
- Rassouli FB, Matin MM, Saeinasab M (2016) Cancer stem cells in human digestive tract malignancies. Tumor Biology 37: 7-21.
- Gong X, Azhdarinia A, Ghosh SC, Xiong W, An Z, Liu Q, et al. (2016) LGR5-targeted antibody-drug conjugate eradicates gastrointestinal tumors and prevents recurrence. Molecular cancer therapeutics. 15: 1580-1590.
- Morita H, Mazerbourg S, Bouley DM, Luo C-W, et al. (2004) Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Molecular and cellular biology 24: 9736-9743.
- Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, et al. (2009) LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Developmental biology. 331: 58-67.
- Yee KK, Li Y, Redding KM, Iwatsuki K, Margolskee RF, et al. (2013) Lgr5‐EGFP marks taste bud stem/progenitor cells in posterior tongue. Stem cells 31: 992-1000.
- Takeda N, Jain R, Li D, Li L, Lu MM, et al. (2013) Lgr5 identifies progenitor cells capable of taste bud regeneration after injury. PLoS One 8: e66314.
- Morrison SJ, Spradling AC (2008) Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132: 598-611.
- Li L, Xie T (2005) Stem cell niche: structure and function. Annu Rev Cell Dev Biol 21: 605-631.
- Huelsken J, Behrens J (2002) The Wnt signaling pathway. Journal of cell science 115: 3977-3978.
- Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4: 68-75.
- Dalley AJ, Majeed AAA, Pitty LP, Major AG, Farah CS (2015) LGR5 expression in oral epithelial dysplasia and oral squamous cell carcinoma. Oral surgery, oral medicine, oral pathology, and oral radiology 119: 436-440.
- Boddupally K, Wang G, Chen Y, Kobielak A (2016) Lgr5 marks neural crest-derived multipotent oral stromal stem cells. Stem cells 34: 720-731.
- Nikitakis NG, Gkouveris I, Aseervatham J, Barahona K, Ogbureke KU (2018) DSPP-MMP20 gene silencing downregulates cancer stem cell markers in human oral cancer cells. Cellular & molecular biology letters 23: 30.
- Morgan R, Mortensson E, Williams A (2018) Targeting LGR5 in Colorectal Cancer: therapeutic gold or too plastic? British journal of cancer 118: 1410.

Figures at a glance